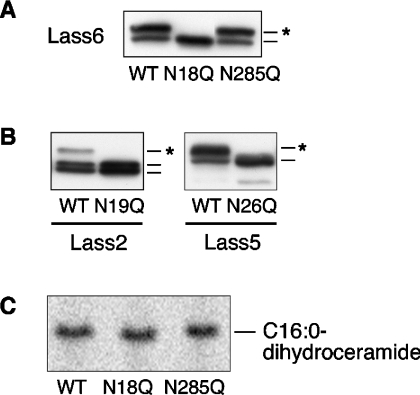
Figure 6. The N-glycosylation site is conserved in Lass2, Lass5 and Lass6.
(A) Identification of the N-glycosylation site of Lass6. HEK 293T cells were transfected with pcDNA3-HA-Lass6 (wild type; WT), pcDNA3-HA-Lass6-N18Q (N18Q) or pcDNA3-HA-Lass6-N285Q (N285Q). Extracts (5 μg of protein) from transfected cells were separated on SDS/PAGE. An asterisk (*) indicates a glycosylated protein. (B) Identification of the conserved N-glycosylation site in Lass2 and Lass5. Extracts (5 μg of protein) for immunoblotting were prepared from HEK 293T cells transfected with each corresponding wild-type (WT) pcDNA3-HA-Lass, or with pcDNA3-HA-Lass2-N19Q (N19Q) or pcDNA3-HA-Lass5-N26Q (N26Q). An asterisk (*) indicates a glycosylated protein. (C) In vitro dihydroceramide synthesis activity of Lass6 mutants. Samples (40 μg of protein) from the same lysates as used in (A) were incubated at 37 °C for 15 min with [3H]dihydrosphingosine/5 μM dihydrosphingosine and 25 μM C16:0-CoA.