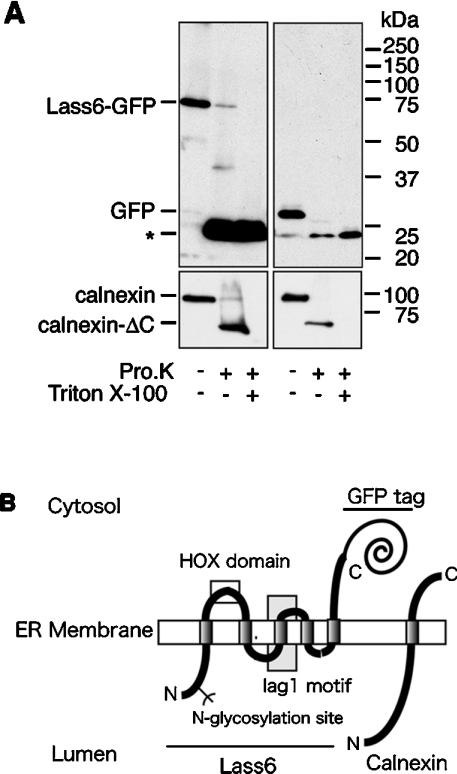
Figure 7. Membrane topology of the Lass6 protein.
(A) Proteinase K digestion assay. Crude membrane fractions were prepared from HEK 293T cells transfected with pEGFP-Lass6 (Lass6–GFP; left panel) or pEGFP-C1 (control vector; right panel). These were untreated or treated with 0.5 mg/ml proteinase K (Pro.K) at 4 °C for 2 h in the absence or presence of 1% Triton X-100. Proteins were separated on an SDS/10%-polyacrylamide gel, followed by immunoblotting with anti-GFP (upper panel) or anti-calnexin (lower panel) antibodies. Calnexin-ΔC is a C-terminally truncated form of calnexin. (B) A model of the proposed topology of Lass6 relative to the established topology of calnexin. Lass6 is proposed to be an internal membrane protein with five transmembrane domains. The HOX (homeobox) domain (clear box) is localized on the cytosolic side of the ER membrane. The highly conserved Lag1 motif contains the putative third transmembrane domain (grey box). The N-glycosylation site (Asn-18) is localized in the N-terminal hydrophilic domain. The N-terminus is located in the lumen of the ER, whereas the C-terminus is located in the cytosol.