Abstract
FGF23 (fibroblast growth factor 23) is a novel phosphaturic factor that influences vitamin D metabolism and renal re-absorption of Pi. The goal of the present study was to characterize the role of the VDR (vitamin D receptor) in FGF23 action using VDR(−/−) (VDR null) mice. Injection of FGF23M (naked DNA encoding the R179Q mutant of human FGF23) into VDR(−/−) and wildtype VDR(+/+) mice resulted in an elevation in serum FGF23 levels, but had no effect on serum calcium or parathyroid hormone levels. In contrast, injection of FGF23M resulted in significant decreases in serum Pi levels, renal Na/Pi co-transport activity and type II transporter protein levels in both groups when compared with controls injected with mock vector or with FGFWT (naked DNA encoding wild-type human FGF23). Injection of FGF23M resulted in a decrease in 25-hydroxyvitamin D 1α-hydroxylase mRNA levels in VDR(−/−) and VDR(+/+) mice, while 25-hydroxyvitamin D 24-hydroxylase mRNA levels were significantly increased in FGF23M-treated animals compared with mock vector control- or FGF23WT-treated animals. The degree of 24-hydroxylase induction by FGF23M was dependent on the VDR, since FGF23M significantly reduced the levels of serum 1,25(OH)2D3 [1,25-hydroxyvitamin D3] in VDR(+/+) mice, but not in VDR(−/−) mice. We conclude that FGF23 reduces renal Pi transport and 25-hydroxyvitamin D 1α-hydroxylase levels by a mechanism that is independent of the VDR. In contrast, the induction of 25-hydroxyvitamin D 24-hydroxylase and the reduction of serum 1,25(OH)2D3 levels induced by FGF23 are dependent on the VDR.
Keywords: fibroblast growth factor 23, kidney, phosphate transport, vitamin D receptor
Abbreviations: ADHR, autosomal dominant hypophosphataemic rickets; BBMV, brush-border membrane vesicle; FGF, fibroblast growth factor; FGF23M, naked DNA encoding the R179Q mutant of human FGF23; FGF23WT, naked DNA encoding wild-type human FGF23; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; 1α(OH)ase, 25-hydroxyvitamin D3 1α-hydroxylase; 24(OH)ase, 25-hydroxyvitamin D 24-hydroxylase; 1,25(OH)2D3, 1,25-dihydroxyvitamin D3; PTH, parathyroid hormone; RT-PCR, reverse transcription–PCR; VDR, vitamin D receptor; XLH, X-linked hypophosphataemia
INTRODUCTION
Pi (inorganic phosphate) is required for cellular function and skeletal mineralization. Pi re-absorption in the renal proximal tubule is a major mechanism in the maintenance of overall Pi homoeostasis; it is a Na+-dependent, secondary active process involving Na/Pi co-transport across the renal brush-border membrane as rate-limiting step, particularly via the Na/Pi co-transporter [1–3]. Mammalian Na/Pi co-transporters have been subdivided into types I–III. The type II Na/Pi co-transporter isoforms (a–c) are the major functional Na/Pi co-transporters [1–3]. The type IIa and IIc co-transporters are expressed in the proximal tubules of the kidney, whereas type IIb is expressed in tissues such as the lung and small intestine [1–3]. Serum phosphate concentrations are maintained within a defined range by expression of type II Na/Pi co-transporters, which is, in turn, regulated by PTH (parathyroid hormone) and vitamin D [1–3]. The actions of vitamin D and PTH are important for the control of intestinal Pi absorption or renal Pi excretion. However, adequate systemic phosphate homoeostasis is likely to require the presence of additional bioactive molecules [1,2].
Studies on patients with tumour-induced osteomalacia and ADHR (autosomal dominant hypophosphataemic rickets) resulted in the identification of FGF23 (fibroblast growth factor 23), a protein that shares sequence identity with other FGFs and which results in hypophosphataemic osteomalacia and inappropriately low serum levels of 1,25(OH)2D3 (1,25-dihydroxyvitamin D3) [3–6]. The FGF23 protein is a secreted protein of 251 amino acids, including a putative N-terminal signal peptide (residues 1–24) [3,4,6]. ADHR is caused by missense mutations at Arg176 and Arg179 of FGF23, which are present in the consensus proteolytic cleavage sequence RXXR [3–6]. Since mutations at Arg176 and Arg179 prevent proteolytic cleavage, a large amount of the mutant protein may escape proteolytic degradation [3,4].
XLH (X-linked hypophosphataemia) is the most common form of inherited rickets, and is caused by inactivating mutations in the PHEX (phosphate regulating endopeptidase homologue, X-linked) gene [3,4]. XLH is characterized by hypophosphataemia due to increased renal phosphate clearance, low or inappropriately normal levels of circulating 1,25(OH)2D3 and rickets/osteomalacia [3,4]. Studies have demonstrated high serum levels of FGF23 in patients with XLH; in addition, levels of FGF23 mRNA expression in bone were significantly increased in the Hyp mouse (which is analogous to the human XLH patient), [7,8]. Thus current evidence indicates that FGF23 may be involved in the pathogenesis of XLH.
Continuous exposure to recombinant FGF23 was shown to cause increased renal Pi clearance resulting from decreased renal expression of type II Na/Pi co-transporters [9–14]. These animals showed paradoxically low/normal 1,25(OH)2D3 levels [9–15]. These reports indicate that FGF23 is an important regulator of Pi homoeostasis and vitamin D metabolism.
Vitamin D plays a central role in modulating Pi homoeostasis and Pi uptake by the small intestine and the kidney [1,2]. It is possible that the inappropriately low levels of 1,25(OH)2D3 may suppress the expression of renal and intestinal Na/Pi co-transporters. Indeed, we demonstrated previously that levels of type IIa and type IIb Na/Pi co-transporter proteins were significantly decreased in VDR(−/−) (vitamin D receptor null) mice [16].
Further, targeted ablation of FGF23 [FGF23(−/−) mice] resulted in increased serum phosphate levels and renal phosphate re-absorption, and an elevation in serum 1,25(OH)2D3 levels secondary to enhanced expression of renal 1α(OH)ase (25-hydroxyvitamin D 1α-hydroxylase). These results indicated that FGF23 is essential for normal phosphate and vitamin D metabolism [17]. In contrast, plasma PTH levels were normal, suggesting that hyperphosphataemia in FGF23(−/−) mice occurs via a PTH-independent mechanism [17]. Shimada et al. [14] suggested that FGF23 suppresses renal 1α(OH)ase expression by co-operating or competing with several humoral factors, such as PTH and 1,25(OH)2D3. Thus, while the mechanisms responsible for the high serum phosphate and 1,25(OH)2D3 levels in FGF23(−/−) mice remain unclear, it is possible that high serum 1,25(OH)2D3 levels stimulate the intestinal absorption and renal re-absorption of Pi via the apical Na/Pi co-transporter. This is supported by the fact that levels of the type IIa Na/Pi co-transporter protein were markedly increased in the apical membranes of renal proximal tubule cells in FGF23(−/−) mice [17].
In our previous studies, we examined the effects of administration of FGF23WT (naked DNA encoding wild-type human FGF23) or FGF23M (naked DNA encoding the R179Q mutant of human FGF23) into rats [12]. Injection of FGF23M into rats resulted in significant decreases in plasma Pi levels, renal Na/Pi co-transport activity and type II Na/Pi co-transporter levels. However, injection of FGF23WT into rats had no significant effects. Rats injected with either FGF23WT or FGF23M highly expressed the human FGF23 transcript in the liver. The levels of plasma human FGF23 protein were markedly increased in rats injected with FGF23M. However, this was not the case in rats injected with FGF23WT [12]. Thus wild-type FGF23 protein may be degraded in the liver or the blood.
The goal of the present study was to use VDR(−/−) mice to determine (i) whether vitamin D is involved in the regulation of renal Pi re-absorption by FGF23, and (ii) whether the VDR is required for the down-regulation of 1α(OH)ase activity by FGF23.
EXPERIMENTAL
Animals and diet
VDR(−/−) mice were generated by gene targeting as described previously [18]. VDR genotypes were confirmed by analysing the DNA obtained from each mouse approx. 3 weeks after birth. Genomic DNA was extracted from tail clippings and amplified by PCR using primers specific for VDR(+/+) exon 2 or the neomycinresistance gene, as described previously [16].
VDR(+/+) and VDR(−/−) mice were weaned at 3 weeks of age, and given free access to water and a control diet containing 0.5% Pi and 0.5% Ca for 5 weeks. After 5 weeks, naked DNA (encoding FGF23 or FGF23 R179Q) or the empty vector was administered by intravenous injection [12,15].
FGF23 mutant construct and injection of naked DNA
DNA encoding human FGF23 or the FGF23 R179Q mutant was subcloned into the pCAGGS3 expression plasmid vector at a unique EcoRI site between the CAG promoter and a 3′-flanking sequence of rabbit β-globin. The empty pCAGGS3 plasmid (kindly provided by Dr J.-i. Miyazaki, Osaka University, Osaka, Japan) was used as a mock control. Next, 1.5 ml of DNA solution containing 10 μg of each expression plasmid [pCGF23 (wild-type human FGF23), pCGFM2 (human FGF23 R179Q mutant) or pCAGGS3 vector] was administered intravenously, as described previously [12,15]. At 4 days after the injection of naked DNA, blood samples were obtained from the abdominal vein, and tissues were rapidly removed under anaesthesia.
Quantitative analysis of FGF23 mRNA
Total RNA was extracted from the livers of transfected animals using ISOGEN (Nippon Gene, Tokyo, Japan), and cDNA was synthesized using M-MLV (Moloney murine leukaemia virus) Reverse H reverse transcriptase (Superscript; Invitrogen) and an oligo(dT)12–18 primer. The amount of human FGF23 cDNA relative to GAPDH (glyceraldehyde-3-phosphate dehydrogenase) cDNA was determined by competitive RT-PCR (reverse transcription–PCR) using a 7700 Sequence Detector (PE Applied Biosystems) [12,15]. The PCR primers used for these experiments did not amplify cDNA of the endogenous mouse FGF23 homologue.
Detection of serum FGF23
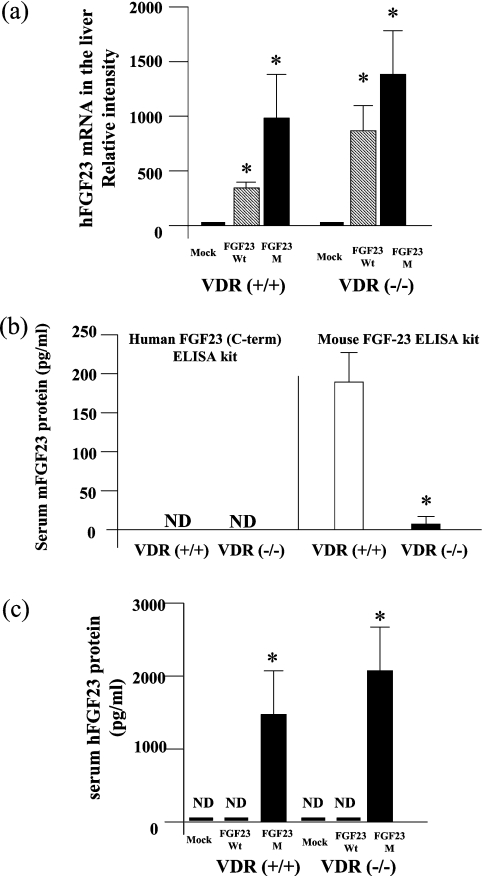
The serum concentration of exogenous human FGF23 in mice was determined using the Human FGF23 (C-term) ELISA kit (Immunotopics, San Clemente, CA, U.S.A.), which only detects human FGF23 [12,15]. The serum concentration of endogenous mouse FGF23 was determined using the FGF-23 ELISA kit (KAINOS Laboratories, Inc., Tokyo, Japan). We analysed the cross-reactivity of FGF23 proteins between mouse and human (see Figure 1b). The human FGF-23 (C-term) ELISA kit did not detect endogenous mouse FGF23 in either VDR(+/+) or VDR(−/−) mice. In contrast, the mouse FGF23 ELISA kit clearly detected endogenous mouse FGF23 in both VDR(+/+) and VDR(−/−) mice. Serum FGF23 protein levels were significantly lower in VDR(−/−) than in VDR(+/+) mice. These results indicated that the human FGF23 (C-term) ELISA kit did not cross-react with the endogenous mouse FGF23.
Figure 1. Expression of FGF23 in VDR(+/+) and VDR(−/−) mice.
At 4 days after administration of FGF23WT, FGF23M or empty vector to VDR(+/+) mice and VDR(−/−) mice, we analysed the expression of human FGF23 mRNA and protein. (a) Expression of FGF23 in the liver, as assessed by competitive RT-PCR (see the Experimental section). FGF23 mRNA levels are shown relative to those of GAPDH mRNA. One-way ANOVA (post hoc Scheffé F-test) and two-factor factorial ANOVA were performed. Values are means±S.E.M. (n=5–6); *P<0.05 compared with mock vector control. (b) Serum concentrations of endogenous mouse FGF23 (mFGF23) protein in VDR(+/+) and VDR(−/−) mice were determined using two separate ELISA kits, as described in the Experimental section. Statistical analysis of endogenous serum FGF23 levels in VDR(−/−) and VDR(+/+) mice was performed using Welch's test. Values are means±S.E.M. (n=10); *P<0.05 for VDR(+/+) compared with VDR(−/−) mice. (c) Serum concentrations of exogenous human FGF23 protein (hFGF23) were determined by ELISA in VDR(+/+) and VDR(−/−) mice injected with naked DNA. One-way ANOVA (post hoc Scheffé F-test) and two-factor factorial ANOVA were performed. Values are means±S.E.M. (n=5–6); *P<0.05 compared with mock vector control. ND, not detected.
Serum calcium, Pi, PTH and 1,25(OH)2D3 levels
The serum concentrations of Ca2+ and Pi were determined by the Calcium-E test and the Phospha-C test (both from Wako, Osaka, Japan) respectively. Serum concentrations of PTH were determined using the mouse PTH ELISA kit (Immunotopics) [16,17]. Serum concentrations of 1α,25(OH)2D3 were determined by a radioreceptor assay (Mitsubishi BCL, Tokyo, Japan) [15].
Northern blot analysis
Poly(A)+ RNA (3 μg/lane) isolated from mouse intestine or kidney was separated on a 1% (w/v) agarose gel in the presence of 2.2 M formaldehyde and blotted on to a Hybond N+ membrane (Amersham Pharmacia Biotech) as described previously [12,16,19]. Specific probes for 1α(OH)ase, 24(OH)ase (25-hydroxyvitamin D 24-hydroxylase) and each Na/Pi co-transporter were labelled with [32P]dCTP using the Megaprime DNA Labeling System (Amersham Pharmacia Biotech) [12,16,19]. The specific probes for 1α(OH)ase and 24(OH)ase were similar to those used for RT-PCR. Hybridization proceeded for 3 h at 65 °C, and the blot was evaluated by autoradiography using a Fujix BAS-1500 bioimaging analyser (Fujifilm, Tokyo, Japan).
RT-PCR for 1α(OH)ase and 24(OH)ase
Kidney total RNA extraction and cDNA synthesis were performed as described above. The PCR primers were designed for 1α(OH)ase and 24(OH)ase as follows: 1α(OH)ase (forward/reverse; 5′-3′), CCGCGGGCTATGCTGGAAC/CTCTGGGCAAAGGCAAACATCTGA; 24(OH)ase (forward/reverse; 5′-3′), TGGGAAGATGATGGTGACCC/ACTGTTCCTTTGGGTAGCGT. PCR were performed for 32 cycles, with cycle conditions of 94 °C for 1 min, 58 °C for 1 min, and 72 °C for 1 min. All amplicons were sequenced to confirm the specificity of amplification.
Preparation of BBMVs (brush-border membrane vesicles) and transport assay
BBMVs were prepared from mouse kidney or intestine by the Ca2+ precipitation method, as described previously [12,16,19]. BBMV 32P uptake was measured by the rapid filtration technique. A sample of 10 μl of vesicle suspension was added to 90 μl of incubation solution (100 mM NaCl, 100 mM mannitol, 20 mM Hepes/Tris and 0.1 mM KH232PO4), and the preparation was incubated at 20 °C. Transport was terminated by rapid dilution with ice-cold saline, and the reaction mixture was transferred immediately to a remoistened filter (0.45 μm) and maintained under a vacuum [12,16].
Immunoblotting
Protein samples were heated at 95 °C for 5 min in sample buffer in the presence of 2-mercaptoethanol and subjected to SDS/PAGE. The separated proteins were transferred by electrophoresis to a Hybond-P PVDF transfer membranes and then treated with diluted affinity-purified antibodies against type IIa (1:4000) or type IIc (1:1000) Na/Pi co-transporters [17,18]. Mouse anti-actin monoclonal antibody (CHEMICON) was used as an internal control. Horseradish peroxidase-conjugated anti-rabbit or anti-mouse IgG was utilized as the secondary antibody (Jackson Immuno-Research Laboratories), and signals were detected using the ECL Plus® system (Amersham Pharmacia Biotech) [12,16,19].
Statistical analysis
One-way ANOVA (post hoc Scheffé F-test) and two-factor factorial ANOVA were performed. Data are expressed as means±S.E.M. Statistical analysis of endogenous serum FGF23 measurements in VDR(−/−) and VDR(+/+) mice was performed using Welch's test. P<0.05 was considered significant.
RESULTS
Effects of FGF23 on food intake in VDR(+/+) and VDR(−/−) mice
VDR(−/−) and VDR(+/+) mice were weaned at 3 weeks of age, housed in plastic cages, and given free access to water (distilled water) and diet containing 0.5% calcium and 0.5% phosphorus for 5 weeks, as described in the Experimental section. We measured the dietary intake of all VDR(−/−) and VDR(+/+) mice used in the study. As shown in Table 1, there were no differences in food intake between VDR(+/+) and VDR(−/−) mice for up to 4 days after the injection of naked DNA.
Table 1. Effects of FGF23 on food intake in VDR(+/+) and VDR(−/−) mice.
One-way ANOVA (post hoc Scheffé F-test) and two-factor factorial ANOVA were performed. Values are means±S.E.M. (n=6–10).
| Food intake (g/day) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Mice | Injection | No. of days of injection… | −1 | 0 | 1 | 2 | 3 | 4 |
| VDR(+/+) | Mock | 4.2±0.8 | 3.6±1.0 | 4.7±0.9 | 3.8±0.9 | 5.3±0.8 | 4.2±0.8 | |
| FGF23WT | 3.9±0.9 | 3.5±1.1 | 4.5±0.6 | 3.7±0.9 | 5.2±0.8 | 3.9±0.9 | ||
| FGF23M | 4.1±0.9 | 3.8±0.9 | 4.8±0.5 | 3.5±0.9 | 4.7±0.5 | 4.1±0.9 | ||
| VDR(−/−) | Mock | 4.5±0.8 | 3.7±1.2 | 4.3±0.8 | 3.8±0.8 | 4.6±0.8 | 3.9±1.2 | |
| FGF23WT | 4.2±0.9 | 3.9±0.9 | 4.7±0.6 | 3.5±1.0 | 4.9±0.8 | 3.6±1.0 | ||
| FGF23M | 4.4±0.8 | 3.6±0.9 | 4.2±0.9 | 4.1±1.1 | 4.7±0.8 | 3.5±0.9 | ||
Expression of mutant FGF23 mRNA and protein
We demonstrated previously that injection of naked DNA plasmids encoding the human FGF23 gene into animals resulted in the expression of FGF23 protein in the liver for at least 4 days [12,15]. To determine if the effect of FGF23 on intestinal and renal phosphate transport is dependent on vitamin D, naked DNA plasmids encoding the human FGF23 gene were injected into VDR(+/+) and VDR(−/−) mice. At 4 days after injection, wild-type human FGF23 and mutant FGF23-R179Q mRNAs were present in liver tissue at relatively high levels in both VDR(+/+) and VDR(−/−) mice, as shown by quantitative PCR (Figure 1a).
Using the human FGF23 (C-term) ELISA kit, we demonstrated that protein levels of human FGF23 were markedly increased in both VDR(+/+) and VDR(−/−) mice injected with FGF23M, but not in mice injected with FGF23WT (Figure 1c).
Effects of FGF23M on serum levels of calcium, Pi, PTH and vitamin D
Serum calcium, Pi, PTH and 1,25(OH)2D3 levels were determined 4 days after injection of FGF23WT, FGF23M or mock vector in VDR(+/+) and VDR(−/−) mice (Table 2). Serum calcium and Pi levels were significantly decreased in VDR(−/−) mice compared with VDR(+/+) mice, as described previously [16,18]. In contrast, serum PTH and 1,25(OH)2D3 levels were markedly increased in VDR(−/−) mice compared with VDR(+/+) mice [16,18]. Injection of FGF23M resulted in a significant decrease in serum Pi, but did not affect serum calcium or PTH, in both VDR(+/+) and VDR(−/−) mice. However, FGF23M resulted in a significant decrease in 1,25(OH)2D3 levels only in VDR(+/+) mice. There were no significant differences in any serum parameter when comparing mice injected with FGF23WT and those injected with mock vector.
Table 2. Effects of FGF23 on serum levels of calcium, Pi, 1,25(OH)2D3 and PTH in VDR(+/+) and VDR(−/−) mice at 4 days after injection.
One-way ANOVA (post hoc Scheffé F-test) and two-factor factorial ANOVA were performed. Values are means±S.E.M. (n=6–10). Significance of differences: *P<0.05 compared with VDR(+/+) (mock); †P<0.005 compared with VDR(+/+) (FGF23WT); ‡P<0.005 compared with VDR(−/−) (mock); §P<0.05 compared with VDR(−/−) (FGF23WT).
| VDR(+/+) | VDR(−/−) | |||||
|---|---|---|---|---|---|---|
| Mock | FGF23WT | FGF23M | Mock | FGF23WT | FGF23M | |
| Calcium (mg/dl) | 9.02±0.14 | 9.03±0.23 | 9.24±0.24 | 6.61±0.52* | 6.90±0.39 | 7.46±0.16 |
| Pi (mg/dl) | 7.43±0.40 | 7.43±0.31 | 5.87±0.16*† | 5.73±0.27* | 5.12±0.30 | 3.59±0.36‡§ |
| 1,25(OH)2D3 (pg/ml) | 82±16 | 130±47 | 6±0.16*† | 630±6* | 617±16 | 610±8 |
| PTH (pg/ml) | 19.3±3.0 | 18.5±2.3 | 16.0±1.0 | 343±25.9* | 281±47.6 | 277±25.3 |
Effects of FGF23M on renal and intestinal Na/Pi transport activity in VDR(+/+) and VDR(−/−) mice
We reported previously that intestinal Na/Pi co-transport activity in VDR(−/−) mice was reduced to 60% of that seen in VDR(+/+) mice, and that expression of intestinal Na/Pi co-transporter (type IIb) protein was markedly suppressed in VDR(−/−) mice when compared with VDR(+/+) mice [16]. Further, whereas renal BBMV Na/Pi co-transport activity was similar when comparing VDR(−/−) mice and VDR(+/+) mice, expression of type IIa Na/Pi protein was slightly and significantly decreased in VDR(−/−) mice, and there was no difference in type IIc Na/Pi protein expression between VDR(−/−) and VDR(+/+) mice [16].
In the present study, injection of FGF23M resulted in a significant reduction in intestinal Na/Pi co-transport activity in VDR(+/+) mice, but had no such effect in VDR(−/−) mice (Table 3). Injection of FGF23M also resulted in a significant reduction in renal Na/Pi co-transport activity in both VDR(+/+) and VDR(−/−) mice.
Table 3. Effects of injection of FGF23M on intestinal and renal Na+-dependent Pi transport activity in VDR(+/+) and VDR(−/−) mice.
Na+-dependent Pi co-transport activity was assessed by the measurement of Pi uptake into intestinal or renal BBMVs. One-way ANOVA (post hoc Scheffé F-test) and two-factor factorial ANOVA were performed. Values are means±S.E.M. (n=5–6). Significance of differences: *P<0.05 compared with mock; †P<0.05 compared with FGF23WT.
| Pi uptake (nmol/30s per mg of protein) | ||||||
|---|---|---|---|---|---|---|
| VDR(+/+) | VDR(−/−) | |||||
| Mock | FGF23WT | FGF23M | Mock | FGF23WT | FGF23M | |
| Intestine | 0.533±0.03 | 0.467±0.070 | 0.304±0.050*† | 0.338±0.040 | 0.364±0.020 | 0.315±0.060 |
| Kidney | 1.024±0.133 | 0.984±0.100 | 0.743±0.040*† | 1.104±0.171 | 0.960±0.161 | 0.733±0.060*† |
Effects of FGF23M on renal type II Na/Pi co-transporter proteins in VDR(+/+) and VDR(−/−) mice
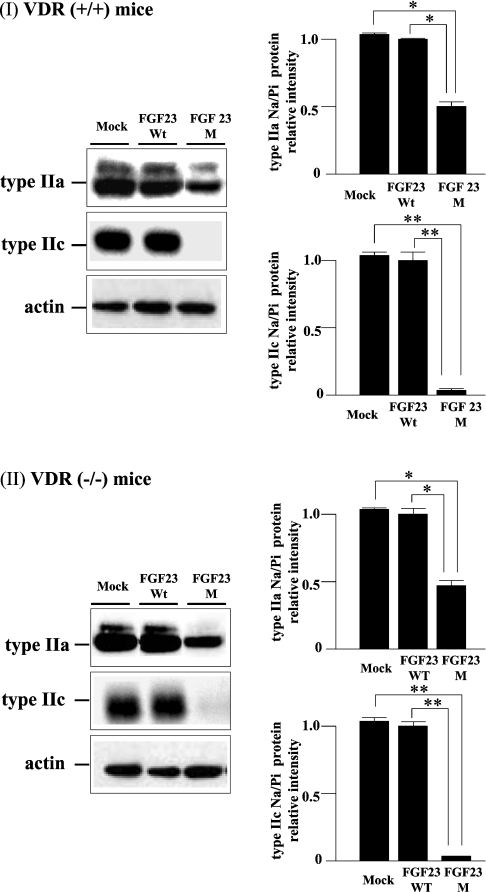
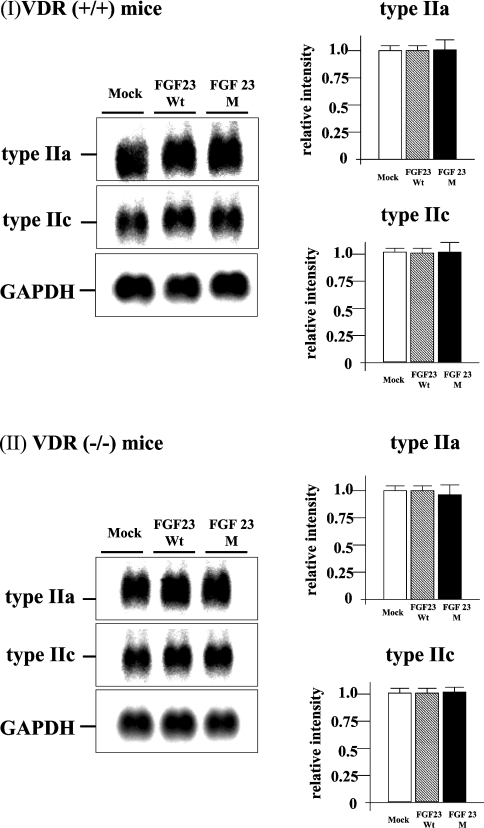
Injection of FGF23M resulted in significant attenuation of type IIa and type IIc renal Na/Pi co-transporter protein expression in both VDR(+/+) and VDR(−/−) mice (Figures 2I and 2II, left panels). As shown in Figure 2 (right panels), the levels of type IIa Na/Pi co-transporter protein in BBMVs were decreased to 50% and those of type IIc protein were markedly reduced, compared with BBMVs from mock vector control-injected animals, in both VDR(+/+) and VDR(−/−) mice. However, injection of FGF23M had no effect on type IIa or type IIc transporter mRNA levels in VDR(+/+) or VDR(−/−) mice (Figure 3). As described previously [12,15], injection of mock vector or FGF23WT had no effect on intestinal or renal Na/Pi co-transport activity, or transporter protein or mRNA levels, in VDR(+/+) or VDR(−/−) mice (Table 2, Figures 2 and 3).
Figure 2. Western blot analysis of renal type II Na/Pi co-transporters.
BBMVs (20 μg/lane) isolated from the kidneys of VDR(+/+) and VDR(−/−) mice injected with empty vector, FGF23WT or FGF23M were loaded into each lane. Upper panels, type IIa co-transporter; middle panels, type IIc Na/Pi co-transporter; lower panels, actin (internal control). The immunoreactive band intensity for mice injected with the empty vector was 1.0. One-way ANOVA (post hoc Scheffé F-test) and two-factor factorial ANOVA were performed. Values are means±S.E.M. (n=5–6); the significance of differences is indicated by *P<0.05 and **P<0.01.
Figure 3. Northern blot analysis of renal Na/Pi co-transporters.
Poly(A)+ RNA was extracted from the kidneys of VDR(+/+) and VDR(−/−) mice that had been injected with FGF23WT, FGF23M or the empty vector. Each lane was loaded with 3 μg of RNA. Upper panels, type IIa co-transporter; middle panels, type IIc Na/Pi co-transporter; lower panels, GAPDH (internal control). The visualized band intensity for the mice injected with the empty vector expression was designated as 1.0, and all other band intensities were expressed relative to this value. One-way ANOVA (post hoc Scheffé F-test) and two-factor factorial ANOVA were performed. Values are means±S.E.M. (n=5–6).
Effects of FGF23 on 1α(OH)ase and 24(OH)ase mRNA levels
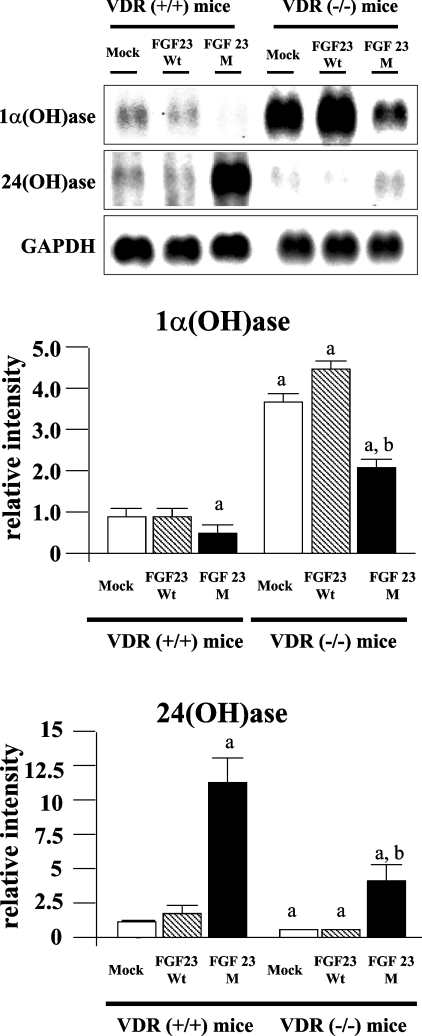
VDR(−/−) mice have higher 1α(OH)ase mRNA levels and lower 24(OH)ase mRNA levels when compared with VDR(+/+) mice [20,21]. Injection of FGF23M resulted in a marked reduction in 1α(OH)ase mRNA levels (Figure 4) and a significant increase in 24(OH)ase mRNA levels (Figure 4) in both VDR(+/+) and VDR(−/−) mice. However, the increase in 24(OH)ase mRNA levels was much less in VDR(−/−) mice than in VDR(+/+) mice.
Figure 4. Effects of injection of FGF23M on 1α(OH)ase and 24(OH)ase mRNA levels.
Poly(A)+ RNA was extracted from the kidneys of VDR(+/+) and VDR(−/−) mice that had been injected with FGF23WT, FGF23M or the empty vector. Each lane was loaded with 3 μg of RNA. Upper panel, 1α(OH)ase; middle panel, 24(OH)ase; lower panel, GAPDH (internal control). In the histograms, the visualized band intensity for the VDR(+/+) mice injected with the empty vector was designated as 1.0, and all other band intensities are expressed relative to this value. One-way ANOVA (post hoc Scheffé F-test) and two-factor factorial ANOVA were performed. Values are means±S.E.M. (n=5–6); ap<0.05 compared with VDR(+/+) mock vector control., bp<0.05 compared with VDR(−/−) mock vector control.
DISCUSSION
The present study used VDR(+/+) and VDR(−/−) mice to investigate whether vitamin D is involved in the FGF23-mediated regulation of renal Pi re-absorption, and whether the VDR is required for the FGF23-mediated down-regulation of 1,25(OH)2D3 levels. The key findings are: (1) VDR may affect the hepatic expression of exogenous FGF23 mRNA, (2) VDR is not involved in the FGF23-mediated suppression of renal Na/Pi co-transport activity, (3) VDR may be involved in the FGF23-mediated down-regulation of intestinal Na/Pi co-transport activity, (4) VDR is involved in the FGF23-mediated induction of renal 24(OH)ase mRNA, and (5) VDR is important in the FGF23-mediated regulation of plasma 1,25(OH)2D3 levels.
Previous studies demonstrated that injection of FGF23WT into rats resulted in an increase in the hepatic expression of FGF23 mRNA, but not in serum FGF23 levels [12]. This lack of a change in serum FGF23 levels may result from protein degradation of wild-type FGF23 in the liver or the circulation, since rats that received FGF23M showed high levels of mRNA in the liver, and serum FGF23 protein levels also increased. Indeed, the present study demonstrated that wild-type FGF23 was degraded in VDR(+/+) and VDR(−/−) mice. Moreover, the expression of both wild-type and mutant FGF23 in the liver was higher in VDR(−/−) than in VDR(+/+) mice (Figure 1a), which suggests that VDR may control the stability of FGF23 mRNA in the liver. Further studies are needed to clarify the mechanisms underlying the hepatic expression of of FGF23.
Recent studies reported that administration of FGF23 resulted in decreases in type II Na/Pi co-transporter mRNA and proteins levels in the kidney [11,12,14], suggesting that FGF23 may affect the transcriptional step of type II transporter synthesis. However, the present study demonstrated that down-regulation of type II Na/Pi co-transporter expression was not dependent on transcriptionally regulated changes in mRNA. Murer et al. [2] found that changes in type II Na/Pi co-transporter mRNA levels were either rather small or occurred only after prolonged stimulation by thyroid hormone (3,3′,5-tri-iodothyronine) and feeding of a low-Pi diet; furthermore, they occurred after changes in specific transporter protein content, suggesting that changes in mRNA represent a phenomenon secondary to the primary event (i.e. down-regulation or up-regulation of brush border type IIa co-transporter expression) [2]. Thus FGF23 may directly modulate trafficking of the transporter from the apical membrane to the intracellular organelles.
Injection of FGF23M decreased intestinal Na/Pi co-transport activity in VDR(+/+) mice, but not in VDR(−/−) mice, which suggests that the action of the mutant FGF23 on intestinal Pi transport is VDR-dependent. Further, VDR(−/−) mice were characterized by hypophosphataemia, hypocalcaemia and high PTH and 1,25(OH)2D3 levels, which may also affect the action of FGF23M. In a previous study, we investigated the effects of FGF23 in hypophosphataemic animals (fed a low-Pi diet) [12]. After injection of naked FGF23 DNA, renal Na/Pi co-transport activity and type II phosphate transporter protein levels were significantly deceased in hypophosphataemic rats [12]. 1α(OH)ase mRNA levels and intestinal Na/Pi co-transport activity were also deceased in those animals. These data suggested that hypophosphataemia itself does not affect the function of FGF23 in VDR(−/−) mice. Further investigations aimed at characterizing the regulation of intestinal Na/Pi co-transporter (type IIb) gene expression by VDR and mutant FGF23 would be of benefit.
FGF23 acts to decrease 1α(OH)ase mRNA levels and increase 24(OH)ase mRNA levels. Renal 1α(OH)ase and 24(OH)ase are regulated by several factors, including PTH, calcium, Pi and 1,25(OH)2D3 [3–5]. In target tissues, 1,25(OH)2D3 exerts most of its biological actions by binding to the VDR, and feedback regulation of 1α(OH)ase gene expression by 1,25(OH)2D3 has been reported [22–24]. Thus VDR may be important in the regulation of 1α(OH)ase and 24(OH)ase mRNAs by FGF23.
In the present study, injection of FGF23M induced a decrease in 1α(OH)ase mRNA and an increase 24(OH)ase mRNA levels in both VDR(+/+) and VDR(−/−) mice. These results suggest that the actions of FGF23 are independent of the VDR-mediated decrease in 1α(OH)ase mRNA and increase in 24(OH)ase mRNA. The lack of a decrease in serum 1,25(OH)2D3 levels in VDR(−/−) mice injected with FGF23M may be for several reasons. First, degradation of 1,25(OH)2D3 may be insufficient in VDR(−/−) mice, as expression of 24(OH)ase, which is required for the inactivation and degradation of vitamin D metabolites, was relatively low in these mice. Secondly, the serum calcium concentration may directly regulate serum 1,25(OH)2D3 levels. Panda et al. [25] studied groups of VDR(−/−) mice exposed to (1) a high-calcium diet, (2) a high-calcium diet plus injection of 1,25(OH)2D3, and (3) a rescue diet (high calcium, high phosphate and high lactose), and showed that only VDR(−/−) mice receiving a rescue diet had normal plasma calcium levels. Further, VDR(−/−) mice had normalized plasma Pi, 1,25(OH)2D3 and PTH concentrations, as well as increased 24(OH)ase mRNA and decreased in 1α(OH)ase mRNA levels [25]. These data suggest that the normalization in serum calcium levels may be related to normalization of serum 1,25(OH)2D3 in mice fed the rescue diet. In the present study, injection of FGF23M did not affect serum calcium levels in either VDR(+/+) or VDR(−/−) mice. Since serum calcium levels remained low in VDR(−/−) mice, high levels of 1,25(OH)2D3 may persist in these mice. Further studies to investigate the FGF23 signalling pathway would be of benefit in clarifying the physiological role of FGF23 in vitamin D metabolism.
In conclusion, the present study has demonstrated that injection of FGF23M lowered renal Pi transport and 1α(OH)ase levels by a mechanism that is independent of the VDR. In contrast, the induction of 24(OH)ase and reduction in serum 1,25(OH)2D3 levels by FGF23 is dependent on the VDR.
Acknowledgments
We thank Miss Kazuyo Shiozawa for technical support. This work was supported by grants from the Ministry of Education, Science, Sports and Culture of Japan (grants 15790430 to H.S. and 11557202 to K.M.) and the 21st Century COE Program, Human Nutritional Science on Stress Control, Tokushima, Japan.
References
- 1.Miyamoto K., Segawa H., Ito M., Kuwahata M. Physiological regulation of renal sodium-dependent phosphate cotransporters. Jpn. J. Physiol. 2004;54:93–102. doi: 10.2170/jjphysiol.54.93. [DOI] [PubMed] [Google Scholar]
- 2.Murer H., Hernando N., Forster I., Biber J. Proximal tubular phosphate reabsorption: molecular mechanisms. Physiol. Rev. 2000;80:1373–1409. doi: 10.1152/physrev.2000.80.4.1373. [DOI] [PubMed] [Google Scholar]
- 3.Tenenhouse H. S., Sabbagh Y. Novel phosphate-regulating genes in the pathogenesis of renal phosphate wasting disorders. Pflugers. Arch. 2002;444:317–326. doi: 10.1007/s00424-002-0839-4. [DOI] [PubMed] [Google Scholar]
- 4.Quarles L. D. FGF23, PHEX, and MEPE regulation of phosphate homeostasis and skeletal mineralization. Am. J. Physiol. Endocrinol. Metab. 2003;285:E1–E9. doi: 10.1152/ajpendo.00016.2003. [DOI] [PubMed] [Google Scholar]
- 5.The ADHR Consortium. Autosomal dominant hypophosphatemic rickets is associated with mutations in FGF23. Nat. Genet. 2000;26:345–348. doi: 10.1038/81664. [DOI] [PubMed] [Google Scholar]
- 6.White K. E., Jonsson K. B., Carn G., Hampson G., Spector T. D., Mannstadt M., Lorenz-Depiereux B., Miyauchi A., Yang I. M., Liunggren O., et al. The autosomal dominant hypophosphatemic rickets (ADHR) gene is a secreted polypeptide overexpressed by tumors that cause phosphate wasting. J. Clin. Endocrinol. Metab. 2001;86:497–500. doi: 10.1210/jcem.86.2.7408. [DOI] [PubMed] [Google Scholar]
- 7.Liu S., Simpson L. G., Xiao Z. S., Burnham C. E., Quales L. D. Regulation of fibroblastic growth factor 23 expression but not degradation by PHEX. J. Biol. Chem. 2003;278:37419–37426. doi: 10.1074/jbc.M304544200. [DOI] [PubMed] [Google Scholar]
- 8.Yamazaki Y., Okazaki R., Shibata M., Hasegawa Y., Satoh K., Tajima T., Takeuchi Y., Fujita T., Nakahara K., Yamashita T., Fukumoto S. Increased circulatory level of biologically active full-length FGF-23 in patients with hypophosphatemic rickets/osteomalacia. J. Clin. Endocrinol. Metab. 2002;87:4957–4960. doi: 10.1210/jc.2002-021105. [DOI] [PubMed] [Google Scholar]
- 9.Bai X. Y., Miao D., Golzman D., Karaplis A. C. The autosomal dominant hypophosphatemic rickets R176Q mutation in fibroblast growth factor 23 resists proteolytic cleavage and enhances in vivo biological potency. J. Biol. Chem. 2003;278:9843–9849. doi: 10.1074/jbc.M210490200. [DOI] [PubMed] [Google Scholar]
- 10.Bai X. Y., Miao D., Li J., Golzman D., Karaplis A. C. Transgenic mice overexpressing human fibroblast growth factor 23 (R176Q) delineate a putative role for parathyroid hormone in renal phosphate wasting disorders. Endocrinology. 2004;145:5269–5279. doi: 10.1210/en.2004-0233. [DOI] [PubMed] [Google Scholar]
- 11.Larsson T., Marsell R., Schipani E., Ohlsson C., Ljunggren O., Tenenhouse H. S., Juppner H., Jonsson K. B. Transgenic mice expressing fibroblast growth factor 23 under the control of the α1(I) collagen promoter exhibit growth retardation, osteomalacia and disturbed phosphate homeostasis. Endocrinology. 2003;145:3087–3094. doi: 10.1210/en.2003-1768. [DOI] [PubMed] [Google Scholar]
- 12.Segawa H., Kawakami E., Kaneko I., Kuwahata M., Ito M., Kusano K., Saito H., Fukushima N., Miyamoto K. Effect of hydrolysis-resistant FGF23-R179Q on dietary phosphate regulation of the renal type-II Na/Pi transporter. Pflugers Arch. 2003;446:585–592. doi: 10.1007/s00424-003-1084-1. [DOI] [PubMed] [Google Scholar]
- 13.Shimada T., Mizutani S., Muto T., Yoneya T., Hino R., Takeda S., Takeuchi Y., Fujita T., Fukumoto S., Yamashita T. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc. Natl. Acad. Sci. U.S.A. 2001;98:6500–6505. doi: 10.1073/pnas.101545198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shimada T., Hasegawa H., Yamazaki Y., Muto T., Hino R., Takeuchi Y., Nakamura K., Fukumoto S., Yamashita T. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J. Bone Miner. Res. 2004;19:429–435. doi: 10.1359/JBMR.0301264. [DOI] [PubMed] [Google Scholar]
- 15.Saito H., Kusano K., Kinosaki M., Ito H., Hirata M., Segawa H., Miyamoto K., Fukushima N. Human fibroblast growth factor-23 mutants suppress Na+-dependent phosphate co-transport activity and 1α,25-dihydroxyvitamin D3 production. J. Biol. Chem. 2003;278:2206–2211. doi: 10.1074/jbc.M207872200. [DOI] [PubMed] [Google Scholar]
- 16.Segawa H., Kaneko I., Yamanaka S., Ito M., Kuwahata M., Inoue Y., Kato S., Miyamoto K. Intestinal Na/Pi cotransporter adaptation to dietary Pi content in vitamin D-receptor (VDR) null mice. Am. J. Physiol. Renal Physiol. 2004;287:F39–F47. doi: 10.1152/ajprenal.00375.2003. [DOI] [PubMed] [Google Scholar]
- 17.Shimada T., Kakitani M., Yamazaki Y., Hasegawa H., Takeuchi Y., Fujita T., Fukumoto S., Tomizuka K., Yamashita T. Targeted ablation of FGF23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J. Clin. Invest. 2004;113:561–568. doi: 10.1172/JCI19081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshizawa T., Hanada Y., Uematsu Y., Takeda S., Sekine K., Yoshihara Y., Kawakami T., Akioka K., Sato H., Ushiyama Y., et al. Mice lacking the vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nat. Genet. 1997;6:391–396. doi: 10.1038/ng0897-391. [DOI] [PubMed] [Google Scholar]
- 19.Ohkido I., Segawa H., Yanagida R., Nakamura M., Miyamoto K. Cloning, gene structure and dietary regulation of the type-IIc Na/Pi cotransporter in the mouse kidney. Pflugers Arch. 2003;446:106–115. doi: 10.1007/s00424-003-1010-6. [DOI] [PubMed] [Google Scholar]
- 20.Li X., Zheng W., Li Y. C. Altered gene expression profile in the kidney of vitamin D receptor knockout mice. J. Cell. Biochem. 2003;89:709–719. doi: 10.1002/jcb.10547. [DOI] [PubMed] [Google Scholar]
- 21.Takeyama K., Kitanaka S., Sato T., Kobori M., Yanagisawa J., Kato S. 25-Hydroxyvitamin D3 1a-hydroxylase and vitamin D synthesis. Science. 1997;277:1827–1830. doi: 10.1126/science.277.5333.1827. [DOI] [PubMed] [Google Scholar]
- 22.Barletta F., Dhawan P., Christakos S. Integration of hormone signaling in the regulation of human 25(OH)D3 24-hydroxylase transcription. Am. J. Physiol. Endocrinol. Metab. 2004;286:E598–E608. doi: 10.1152/ajpendo.00214.2003. [DOI] [PubMed] [Google Scholar]
- 23.Christakos S., Dhawan P., Liu Y., Peng X., Porta A. New insights into the mechanisms of vitamin D action. J. Cell. Biochem. 2003;88:695–705. doi: 10.1002/jcb.10423. [DOI] [PubMed] [Google Scholar]
- 24.Christakos S., Barletta F., Huening M., Dhawan P., Liu Y., Porta A., Peng X. Vitamin D target proteins: function and regulation. J. Cell. Biochem. 2003;88:238–244. doi: 10.1002/jcb.10349. [DOI] [PubMed] [Google Scholar]
- 25.Panda D. K., Miao D., Bolivar I., Li J., Huo R., Hendy G. N., Goltzman D. Inactivation of the 25-hydroxyvitamin D 1alpha-hydroxylase and vitamin D receptor demonstrates independent and interdependent effects of calcium and vitamin D on skeletal and mineral homeostasis. J. Biol. Chem. 2004;279:16754–16766. doi: 10.1074/jbc.M310271200. [DOI] [PubMed] [Google Scholar]