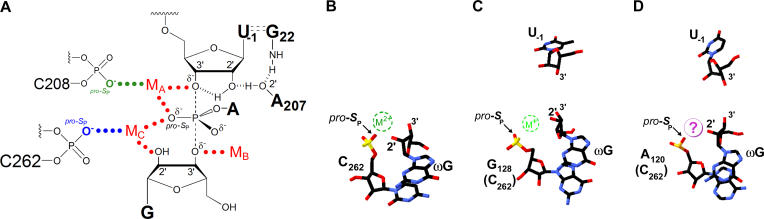
Figure 8. Functional and Structural Models of Group I Intron Active Sites.
(A) Model of the Tetrahymena ribozyme transition state from functional data with the C262 pro-S P phosphoryl oxygen and C208 pro-S P oxygen coordinating to MC and MA, respectively ([35,36,53,58,71,72, 74], data herein, and AVK, JLH, JAP, and DH, unpublished results).
(B) Model of Mg2+ binding in the crystal structure of a thermostable variant of the Tetrahymena group I ribozyme (derived from PDB file 1X8W) [14]. The model shown is derived from molecule C, although the position of the metal ion appears to vary among the four molecules observed in the asymmetric unit. The putative Mg2+ ion (dashed green circle) is 2.4 Å from the pro-S P oxygen of C262 and 2.1 Å from the terminal G (ωG) 2′-OH. U(-1) is not shown, as the crystallized form of the thermostable Tetrahymena variant ribozyme lacks this nucleotide.
(C) Model of K+ binding from the crystal structure of the Azoarcus group I ribozyme (derived from PDB file 1T42) [15]. The putative K+ ion (dashed green circle) is 2.4 Å from the pro-S P oxygen of G128 (C262 homologue) and 2.8 Å from the modeled position for the terminal G (ωG) 2′-OH; the Azoarcus intron construct that was crystallized contained 2′-deoxyguanosine at ωG, and electron density for the K+ ion is observed only in the presence of this 2′-deoxyguanosine modification at ωG.
(D) Model of proposed Mg2+ binding site derived from the crystal structure of the Twort group I ribozyme [13]. The crystallographic data lack clear density for a metal ion in the region expected for the MC binding site but show the pro-S P phosphoryl oxygen of A120 (C262 homologue) and the 2′-OH of ωG appropriately juxtaposed to coordinate a single Mg2+ ion (purple circle).