Abstract
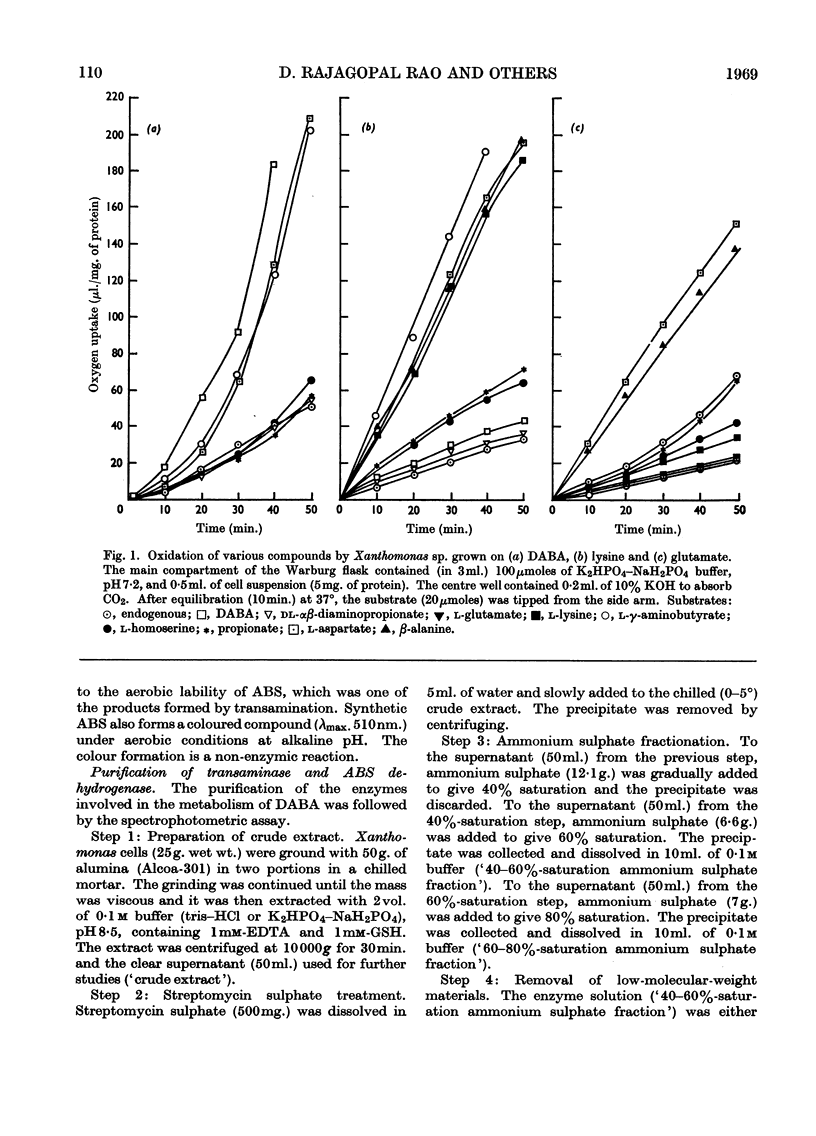
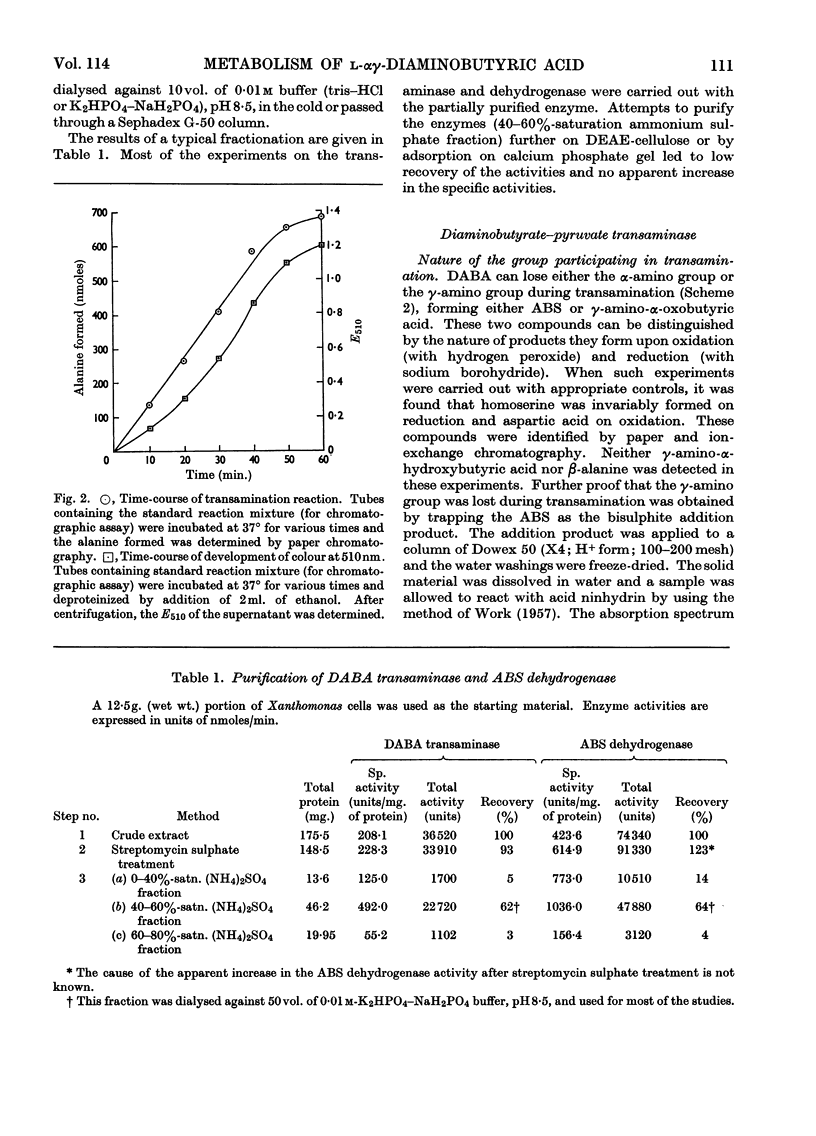
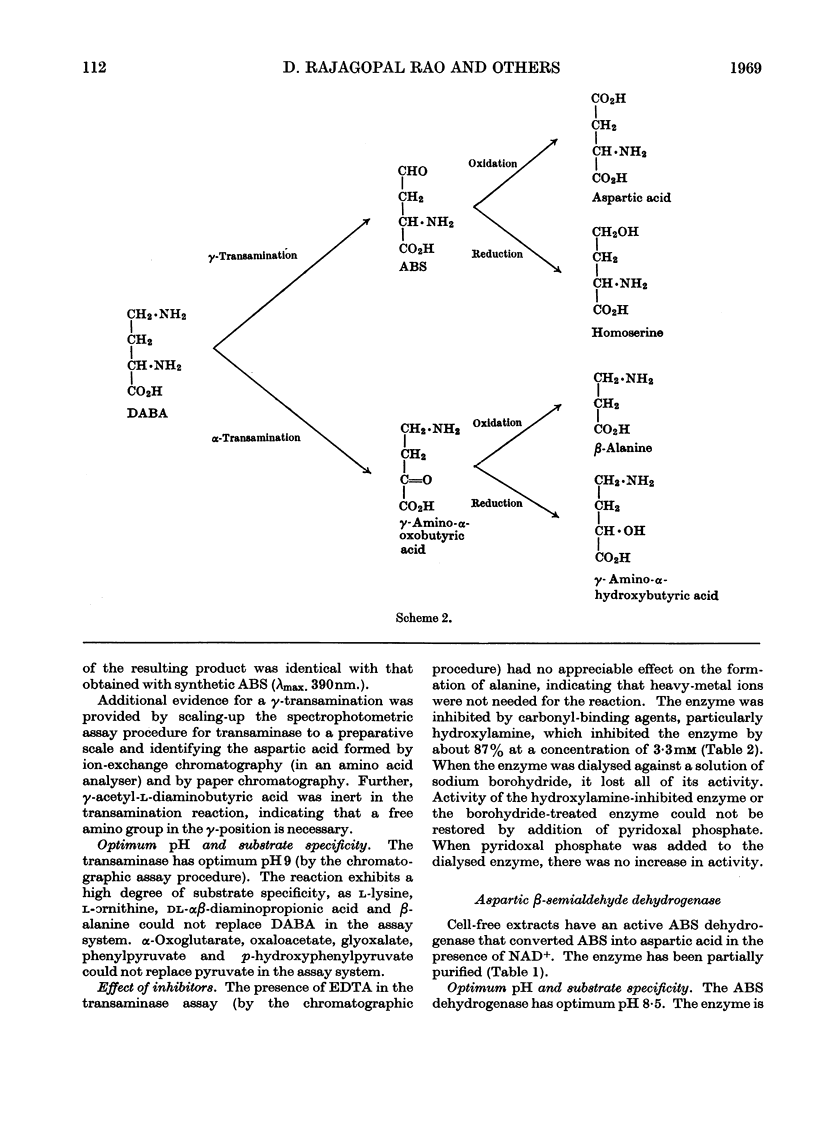
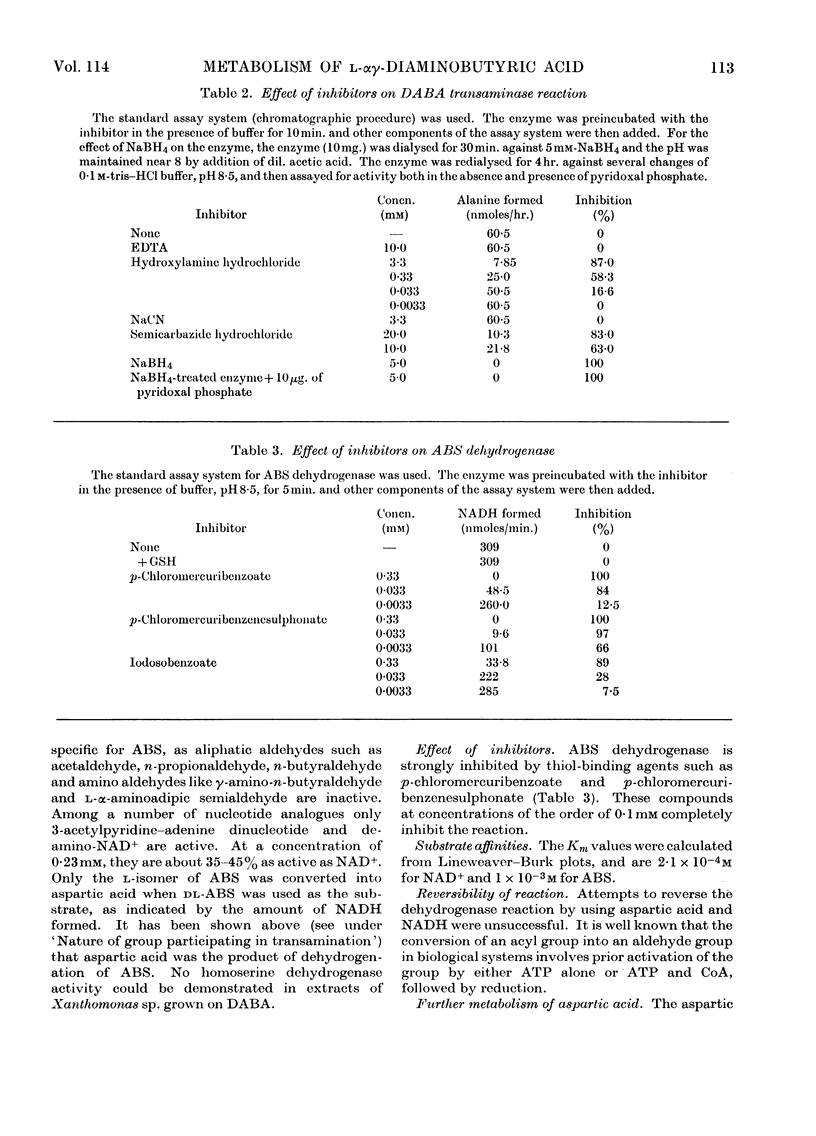
1. l-αγ-Diaminobutyric acid is metabolized in Xanthomonas sp. to aspartic β-semialdehyde, aspartic acid and oxaloacetic acid. 2. Aspartic β-semialdehyde is formed from diaminobutyric acid by a pyruvate-dependent γ-transamination. 3. The transaminase has a pH optimum of 9 and exhibits a high degree of substrate specificity, as analogues of diaminobutyric acid and pyruvate are inert in the system. The transaminase is inhibited by carbonyl-binding agents such as hydroxylamine. 4. Aspartic acid is formed from aspartic β-semialdehyde by an NAD+-dependent dehydrogenation. 5. The dehydrogenase has a pH optimum of 8·5 and is a thiol enzyme. It is specific for aspartic β-semialdehyde but analogues of NAD+ such as 3-acetylpyridine–adenine dinucleotide and deamino-NAD are partly active in the system. 6. The significance of these reactions is discussed in relation to diaminobutyric acid metabolism in plants and mammalian systems.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADAMS E., GOLDSTONE A. Hydroxyproline metabolism. IV. Enzymatic synthesis of gamma-hydroxyglutamate from Delta 1-pyrroline-3-hydroxy-5-carboxylate. J Biol Chem. 1960 Dec;235:3504–3512. [PubMed] [Google Scholar]
- BASSO L. V., RAO D. R., RODWELL V. W. Metabolism of pipecolic acid in a Pseudomonas species. II. delta1-Piperideine-6-carboxylic acid and alpha-aminoadipic acid-delta-semial-dehyde. J Biol Chem. 1962 Jul;237:2239–2245. [PubMed] [Google Scholar]
- BELL E. A. alpha,gamma-Diaminobutyric acid in seeds of twelve species of Lathyrus and identification of a new natural amino-acid, L-homoarginine, in seeds of other species toxic to man and domestic animals. Nature. 1962 Mar 17;193:1078–1079. doi: 10.1038/1931078b0. [DOI] [PubMed] [Google Scholar]
- BLACK S., WRIGHT N. G. Aspartic beta-semialdehyde dehydrogenase and aspartic beta-semialdehyde. J Biol Chem. 1955 Mar;213(1):39–50. [PubMed] [Google Scholar]
- Bell E. A., Tirimanna A. S. Associations of amino acids and related compounds in the seeds of forty-seven species of Vicia: their taxonomic and nutritional significance. Biochem J. 1965 Oct;97(1):104–111. doi: 10.1042/bj0970104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodhue C. T., Snell E. E. The bacterial degradation of pantothenic acid. I. Over-all nature of the reaction. Biochemistry. 1966 Feb;5(2):393–398. doi: 10.1021/bi00866a001. [DOI] [PubMed] [Google Scholar]
- HUGHES D. E. A press for disrupting bacteria and other micro-organisms. Br J Exp Pathol. 1951 Apr;32(2):97–109. [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MUSHAHWAR I. K., KOEPPE R. E. Concerning the metabolism of D- and L-alpha, gamma-diaminobutyric acid-2-C14 in rats. J Biol Chem. 1963 Jul;238:2460–2463. [PubMed] [Google Scholar]
- Nigam S. N., Ressler C. Biosynthesis of 2,4-diaminobutyric acid from L-[3H]homoserine and DL-[1-14C]aspartic acid in Lathyrus sylvestris W. Biochemistry. 1966 Nov;5(11):3426–3431. doi: 10.1021/bi00875a006. [DOI] [PubMed] [Google Scholar]
- O'Neal R. M., Chen C. H., Reynolds C. S., Meghal S. K., Koeppe R. E. The 'neurotoxicity' of L-2,4-diaminobutyric acid. Biochem J. 1968 Feb;106(3):699–706. doi: 10.1042/bj1060699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAULUS H., GRAY E. THE BIOSYNTHESIS OF POLYMYXIN B BY GROWING CULTURES OF BACILLUS POLYMYXA. J Biol Chem. 1964 Mar;239:865–871. [PubMed] [Google Scholar]
- PERKINS H. R., CUMMINS C. S. CHEMICAL STRUCTURE OF BACTERIAL CELL WALLS. ORNITHINE AND 2,4-DIAMINOBUTYRIC ACID AS COMPONENTS OF THE CELL WALLS OF PLANT PATHOGENIC CORYNEBACTERIA. Nature. 1964 Mar 14;201:1105–1107. doi: 10.1038/2011105a0. [DOI] [PubMed] [Google Scholar]
- RESSLER C., REDSTONE P. A., ERENBERG R. H. Isolation and identification of a neuroactive factor from Lathyrus latifolius. Science. 1961 Jul 21;134(3473):188–190. doi: 10.1126/science.134.3473.188. [DOI] [PubMed] [Google Scholar]
- ROTHMAN F., HIGA A. A new two-dimensional system for the separation of amino acids on paper. Anal Biochem. 1962 Mar;3:173–177. doi: 10.1016/0003-2697(62)90052-0. [DOI] [PubMed] [Google Scholar]
- WORK E. Reaction of ninhydrin in acid solution with straight-chain amino acids containing two amino groups and its application to the estimation of alpha epsilon-diaminopimelic acid. Biochem J. 1957 Nov;67(3):416–423. doi: 10.1042/bj0670416. [DOI] [PMC free article] [PubMed] [Google Scholar]


