Abstract
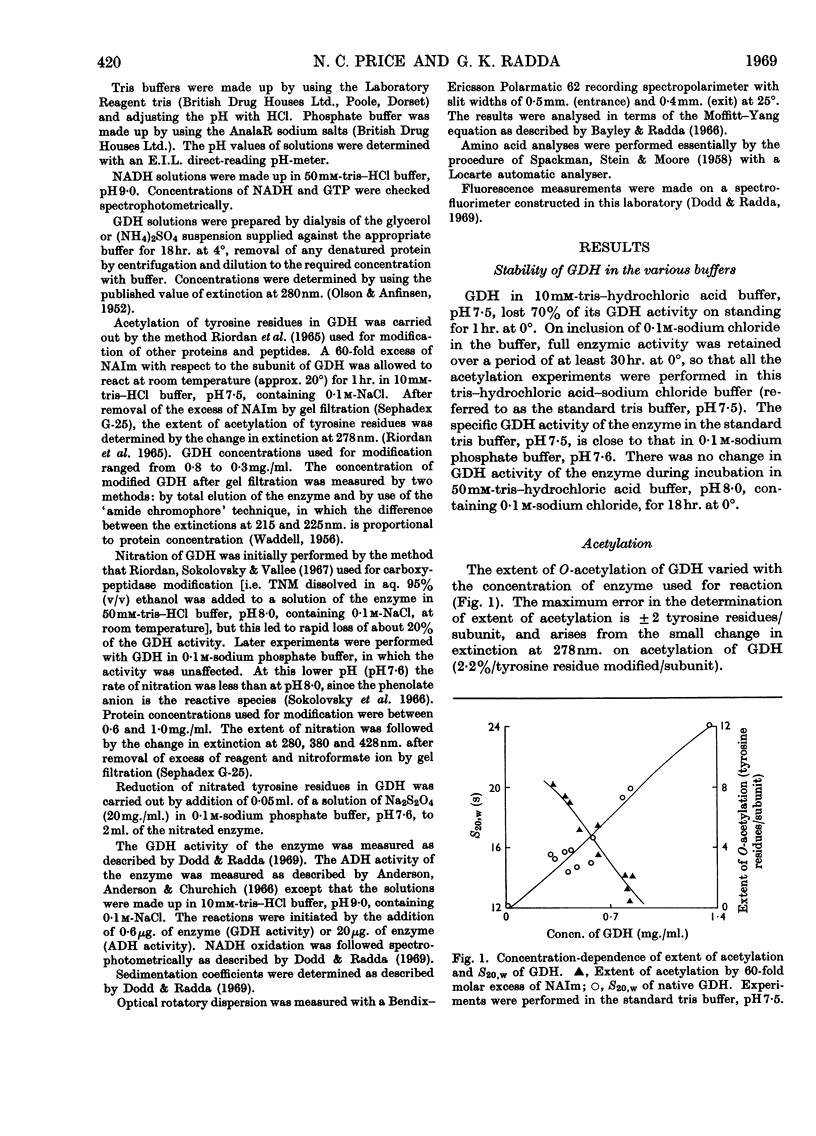
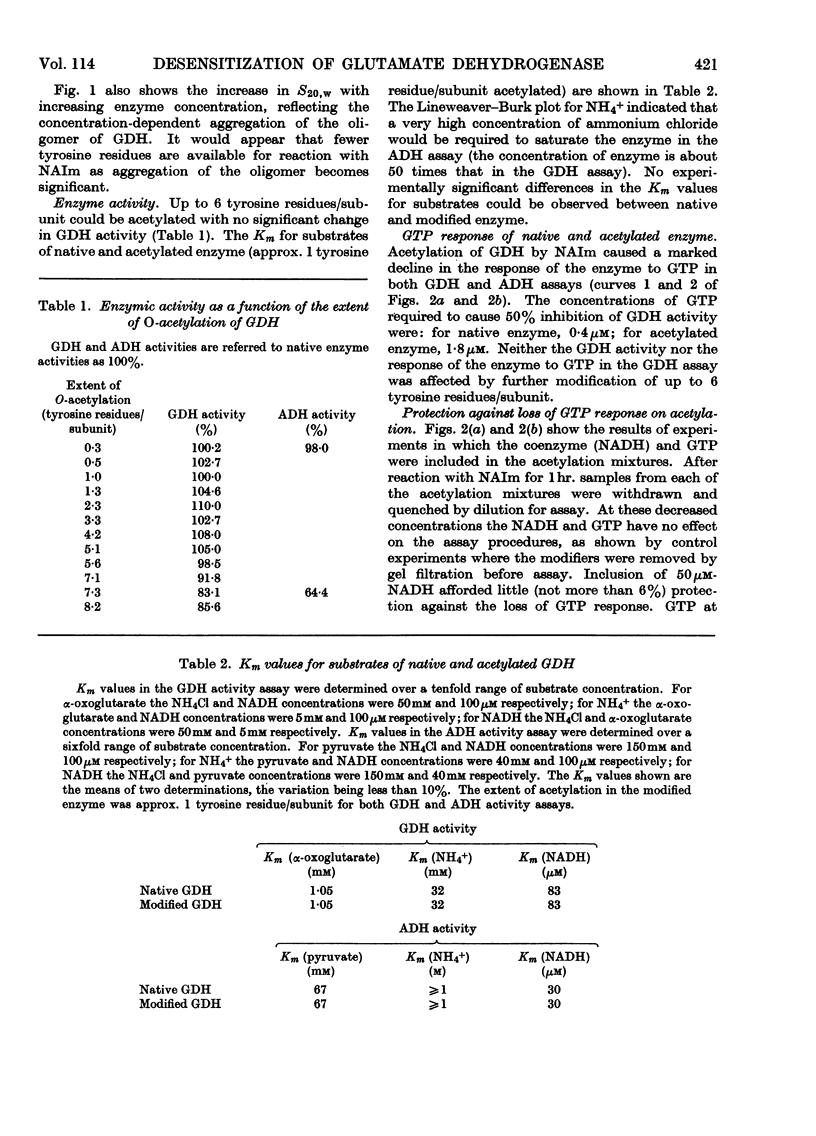
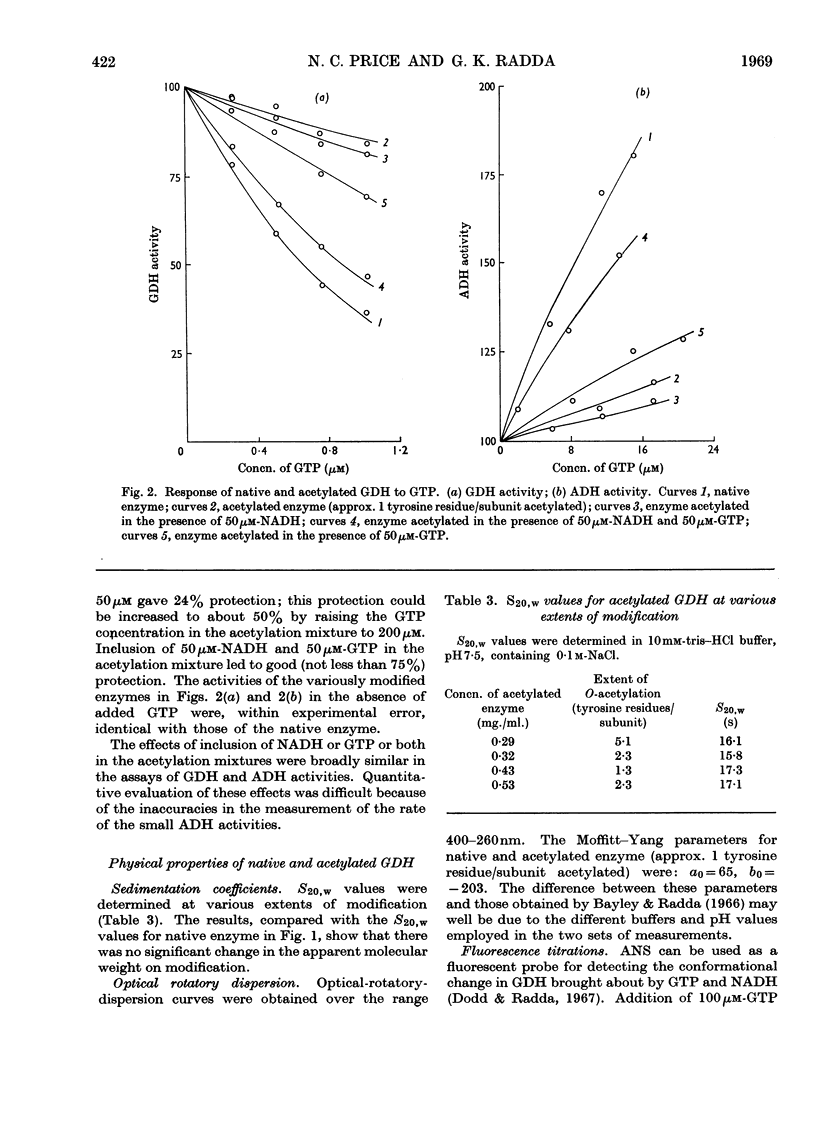
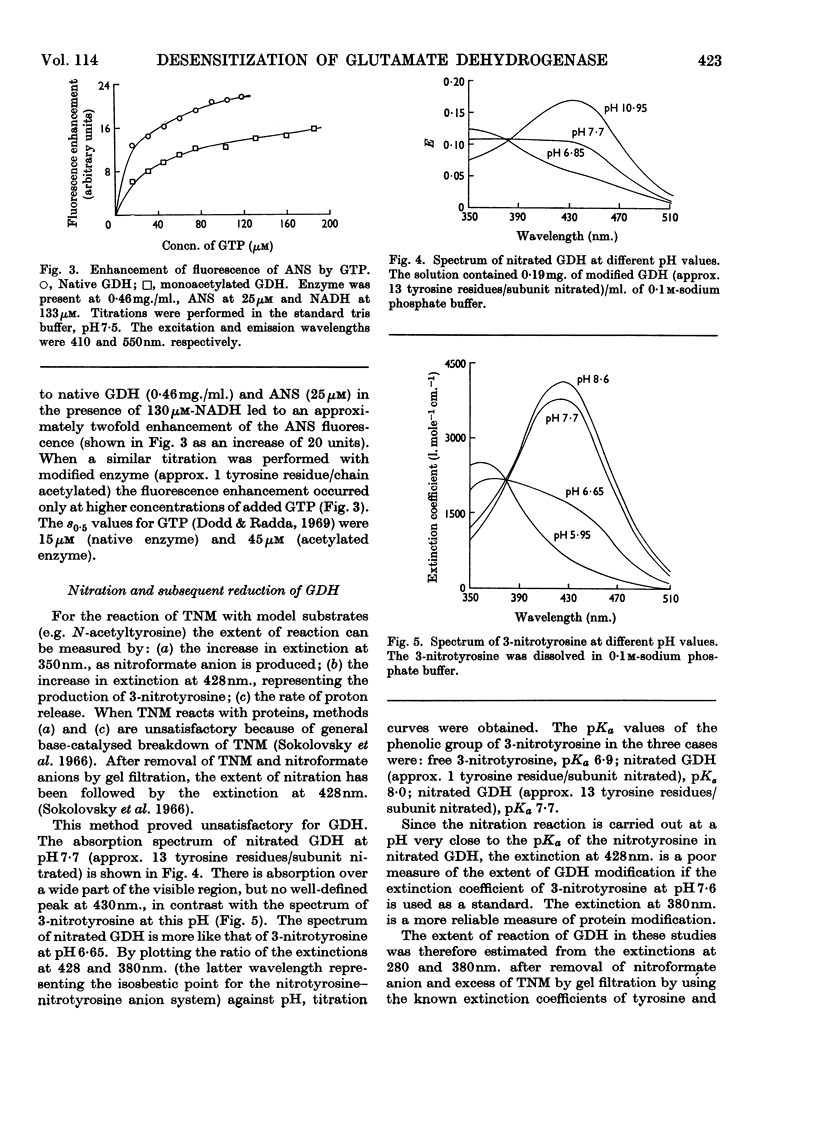
1. The reaction of glutamate dehydrogenase with N-acetylimidazole and with tetranitromethane leads to modification of tyrosine residues. 2. Modification of 1 tyrosine residue/subunit does not affect the enzymic activity but decreases the response of the enzyme to the allosteric inhibitor, GTP. 3. The physical properties of the enzyme (sedimentation coefficient and optical rotatory dispersion) remain unaltered. 4. GTP partially protects against desensitization. 5. The diminished responses of the modified enzymes to GTP are also detected by using the fluorescence of 1-anilinonaphthalene-8-sulphonate as a conformational probe. 6. Difficulties that generally arise in chemical modifications from inhomogeneous distributions of products are discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson B. M., Anderson C. D., Churchich J. E. Inhibition of glutamic dehydrogenase by pyridoxal 5'-phosphate. Biochemistry. 1966 Sep;5(9):2893–2900. doi: 10.1021/bi00873a017. [DOI] [PubMed] [Google Scholar]
- BITENSKY M. W., YIELDING K. L., TOMKINS G. M. THE REVERSAL BY ORGANIC MERCURIALS OF "ALLOSTERIC" CHANGES IN GLUTAMATE DEHYDROGENASE. J Biol Chem. 1965 Feb;240:668–673. [PubMed] [Google Scholar]
- Bayley P. M., Radda G. K. Conformational changes and the regulation of glutamate-dehydrogenase activity. Biochem J. 1966 Jan;98(1):105–111. doi: 10.1042/bj0980105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman A., Sanner T. Pihl A: Modification of regulatory properties of phosphofructokinase acetylation. Eur J Biochem. 1969 Feb;7(4):588–593. doi: 10.1111/j.1432-1033.1969.tb19647.x. [DOI] [PubMed] [Google Scholar]
- Colman R. F., Frieden C. On the role of amino groups in the structure and function of glutamate dehydrogenase. I. Effect of acetylation on catalytic and regulatory properties. J Biol Chem. 1966 Aug 25;241(16):3652–3660. [PubMed] [Google Scholar]
- Cross D. G., Fisher H. F. The spatial location of chromophoric residues in L-glutamate dehydrogenase. Biochemistry. 1966 Mar;5(3):880–885. doi: 10.1021/bi00867a011. [DOI] [PubMed] [Google Scholar]
- Di Prisco G. Desensitization of the allosteric sites of glutamate dehydrogenase by fluorodinitrobenzene. Biochem Biophys Res Commun. 1967 Jan 23;26(2):148–152. doi: 10.1016/0006-291x(67)90226-4. [DOI] [PubMed] [Google Scholar]
- Dodd G. H., Radda G. K. Interaction of glutamate dehydrogenase with fluorescent dyes. Biochem Biophys Res Commun. 1967 May 25;27(4):500–504. doi: 10.1016/s0006-291x(67)80014-7. [DOI] [PubMed] [Google Scholar]
- Doddgh, Radda G. K. 1-Anilinonaphthalene-8-sulphonate, a fluorescent conformational probe for glutamate dehydrogenase. Biochem J. 1969 Sep;114(2):407–417. doi: 10.1042/bj1140407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIEDEN C. GLUTAMATE DEHYDROGENASE. V. THE RELATION OF ENZYME STRUCTURE TO THE CATALYTIC FUNCTION. J Biol Chem. 1963 Oct;238:3286–3299. [PubMed] [Google Scholar]
- Freedman R. B., Radda G. K. The reaction of 2,4,6-trinitrobenzenesulphonic acid with amino acids, Peptides and proteins. Biochem J. 1968 Jul;108(3):383–391. doi: 10.1042/bj1080383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HORINISHI H., HACHIMORI Y., KURIHARA K., SHIBATA K. STATES OF AMINO ACID RESIDUES IN PROTEINS. 3. HISTIDINE RESIDUES IN INSULIN, LYSOZYME, ALBUMIN AND PROTEINASES AS DETERMINED WITH A NEW REAGENT OF DIAZO-I-H-TETRAZOLE. Biochim Biophys Acta. 1964 Jun 8;86:477–489. [PubMed] [Google Scholar]
- KURIHARA K., HORINISHI H., SHIBATA K. REACTIONS OF CYANURIC HALIDES WITH PROTEINS. I. BOUND TYROSINE RESIDUES OF INSULIN AND LYSOZYME AS IDENTIFIED WITH CYANURIC FLUORIDE. Biochim Biophys Acta. 1963 Sep 10;74:678–687. doi: 10.1016/0006-3002(63)91419-7. [DOI] [PubMed] [Google Scholar]
- Koshland D. E., Jr, Némethy G., Filmer D. Comparison of experimental binding data and theoretical models in proteins containing subunits. Biochemistry. 1966 Jan;5(1):365–385. doi: 10.1021/bi00865a047. [DOI] [PubMed] [Google Scholar]
- MONOD J., CHANGEUX J. P., JACOB F. Allosteric proteins and cellular control systems. J Mol Biol. 1963 Apr;6:306–329. doi: 10.1016/s0022-2836(63)80091-1. [DOI] [PubMed] [Google Scholar]
- Malcolm A. D., Radda G. K. Allosteric transitions of glutamate dehydrogenase. Nature. 1968 Aug 31;219(5157):947–949. doi: 10.1038/219947a0. [DOI] [PubMed] [Google Scholar]
- OLSON J. A., ANFINSEN C. B. The crystallization and characterization of L-glutamic acid dehydrogenase. J Biol Chem. 1952 May;197(1):67–79. [PubMed] [Google Scholar]
- Pontremoli S., Grazi E., Accorsi A. Fructose diphosphatase from rabbit liver. VII. Tyrosine residues and adenosine monophosphate inhibition. Biochemistry. 1966 Nov;5(11):3568–3574. doi: 10.1021/bi00875a027. [DOI] [PubMed] [Google Scholar]
- Riordan J. F., Sokolovsky M., Vallee B. L. Environmentally sensitive tyrosyl residues. Nitration with tetranitromethane. Biochemistry. 1967 Jan;6(1):358–361. doi: 10.1021/bi00853a053. [DOI] [PubMed] [Google Scholar]
- Sokolovsky M., Riordan J. F., Vallee B. L. Conversion of 3-nitrotyrosine to 3-aminotyrosine in peptides and proteins. Biochem Biophys Res Commun. 1967 Apr 7;27(1):20–25. doi: 10.1016/s0006-291x(67)80033-0. [DOI] [PubMed] [Google Scholar]
- Sokolovsky M., Riordan J. F., Vallee B. L. Tetranitromethane. A reagent for the nitration of tyrosyl residues in proteins. Biochemistry. 1966 Nov;5(11):3582–3589. doi: 10.1021/bi00875a029. [DOI] [PubMed] [Google Scholar]
- Sokolovsky M., Vallee B. L. The reaction of diazonium-1H-tetrazole with proteins. Determination of tyrosine and histidine content. Biochemistry. 1966 Nov;5(11):3574–3581. doi: 10.1021/bi00875a028. [DOI] [PubMed] [Google Scholar]
- Tomkins G. M., Yielding K. L., Curran J. F., Summers M. R., Bitensky M. W. The dependence of the substrate specificity on the conformation of crystalline glutamate dehydrogenase. J Biol Chem. 1965 Oct;240(10):3793–3798. [PubMed] [Google Scholar]
- WADDELL W. J. A simple ultraviolet spectrophotometric method for the determination of protein. J Lab Clin Med. 1956 Aug;48(2):311–314. [PubMed] [Google Scholar]


