Abstract
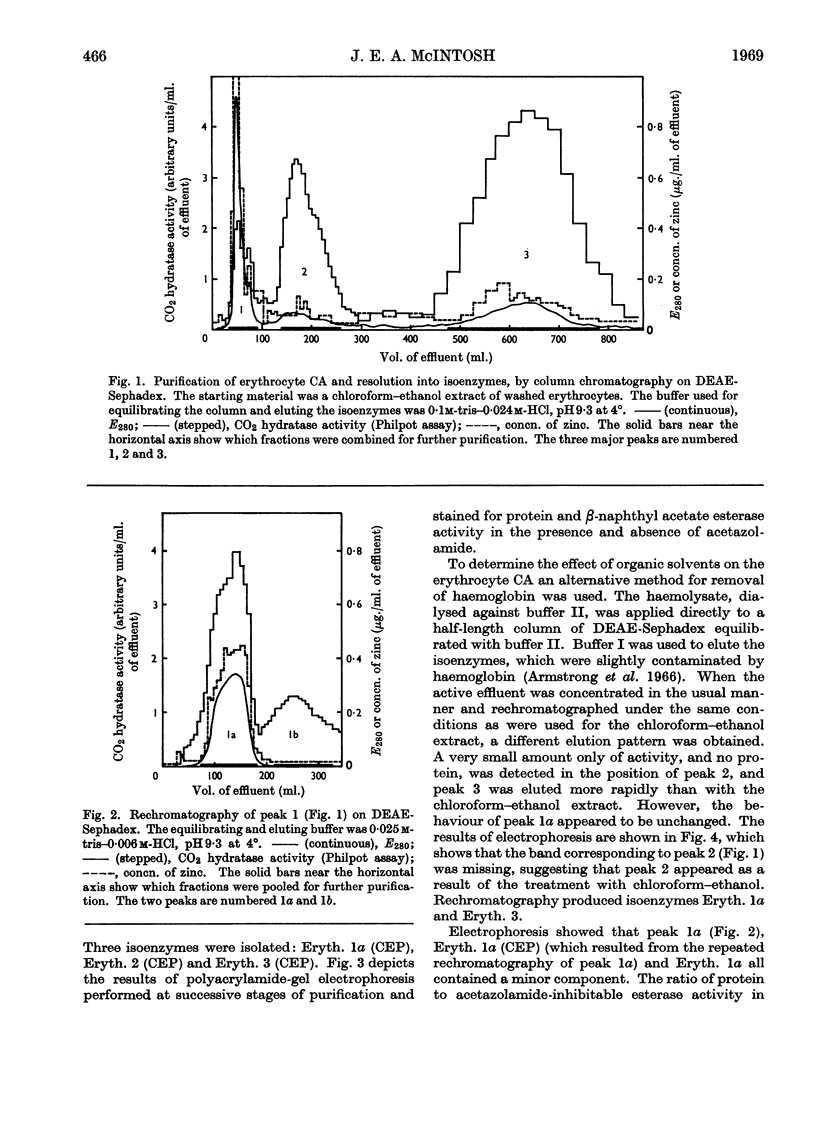
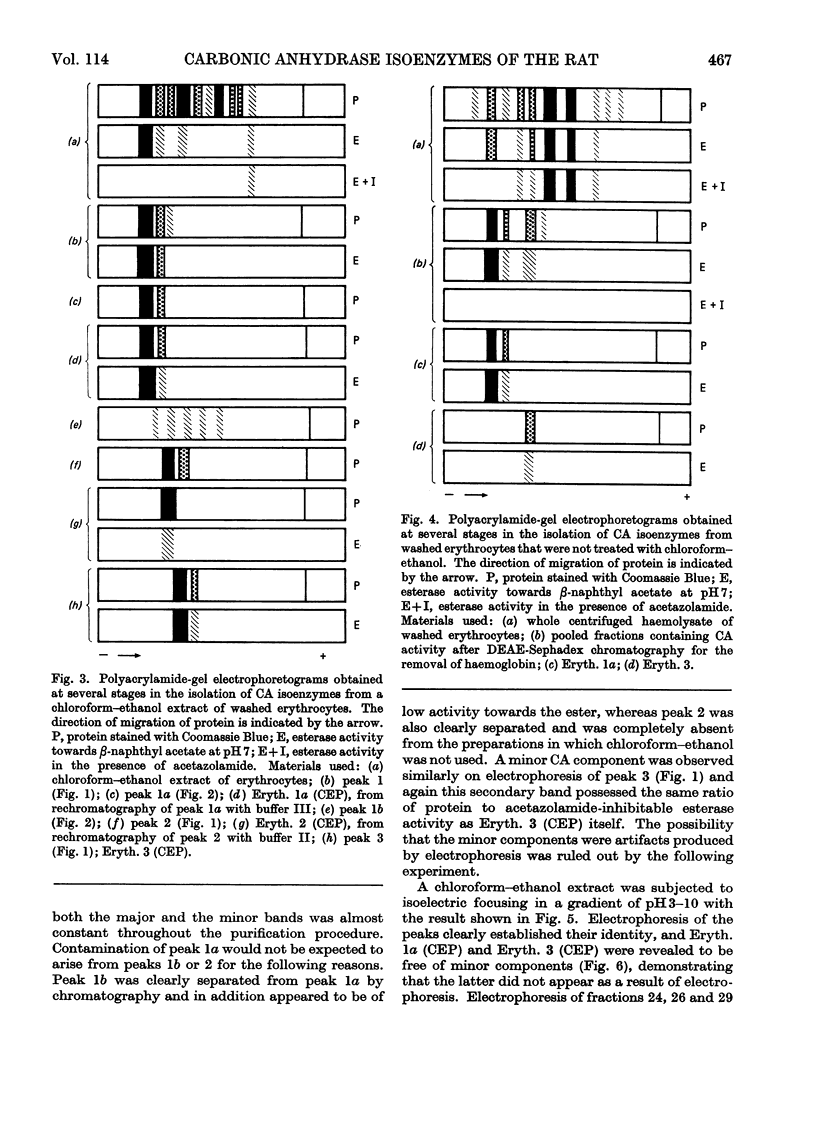
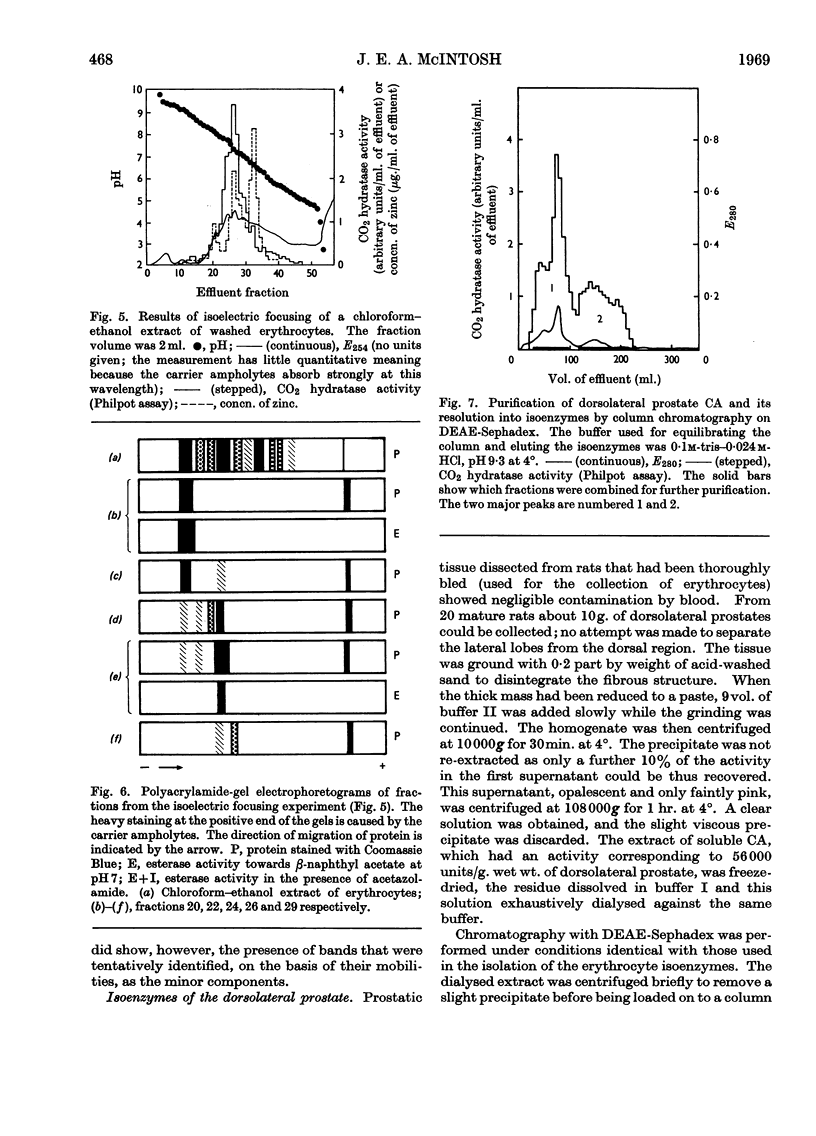
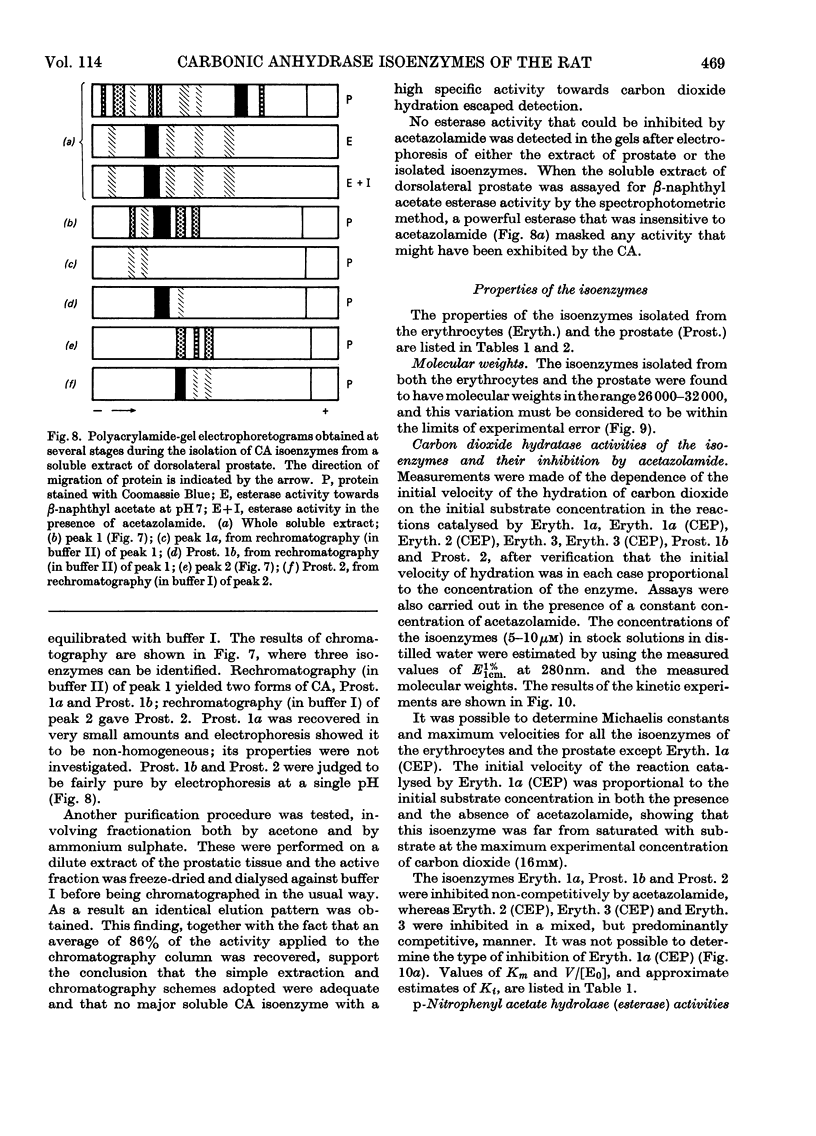
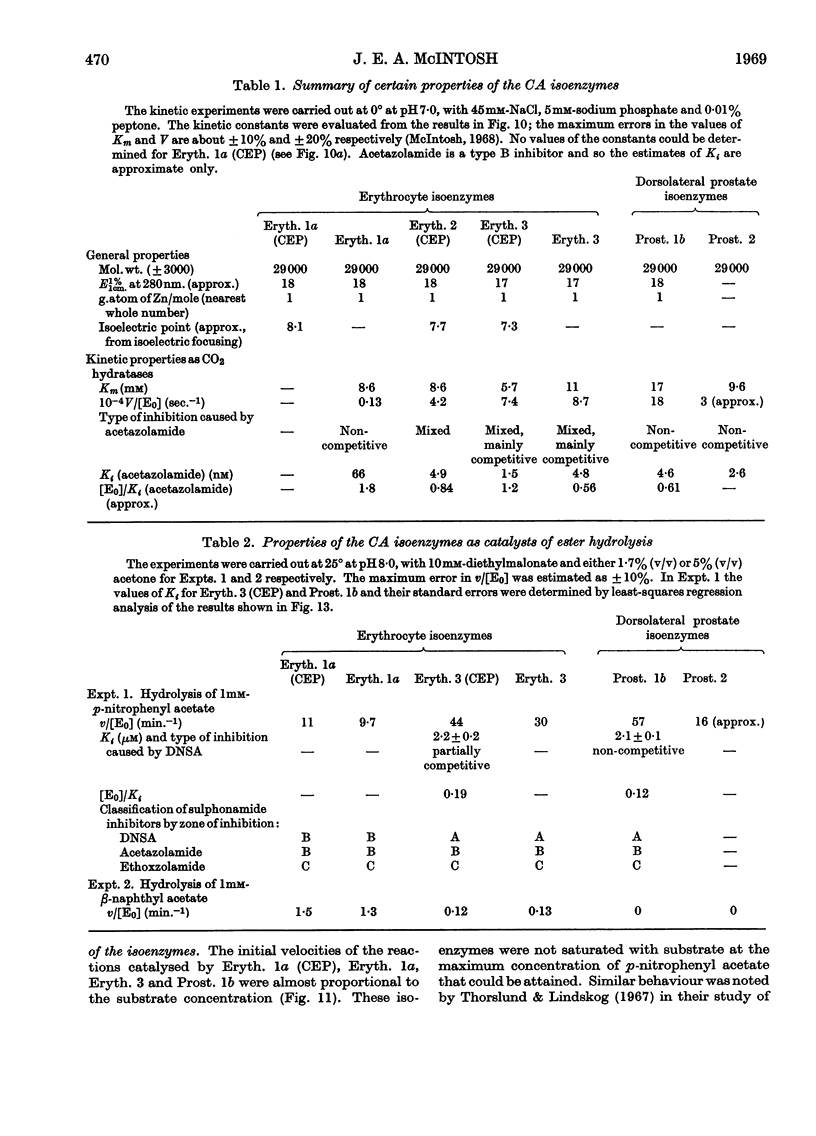
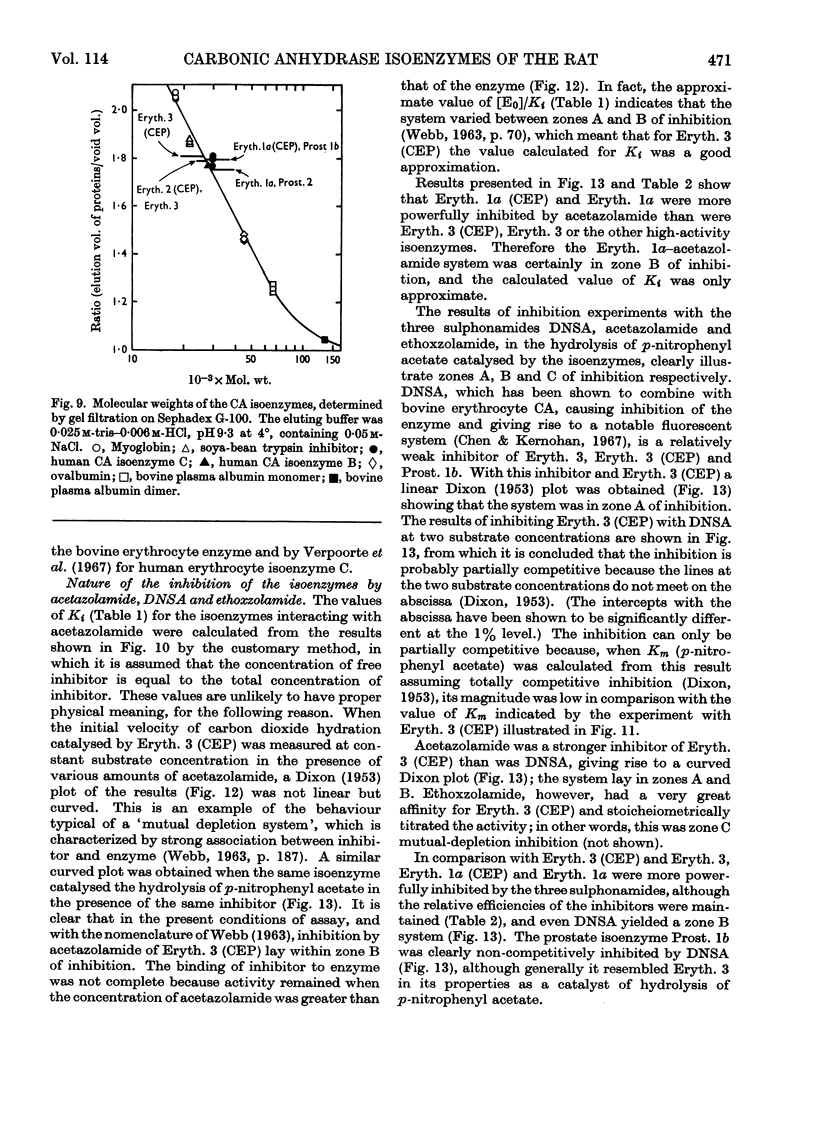
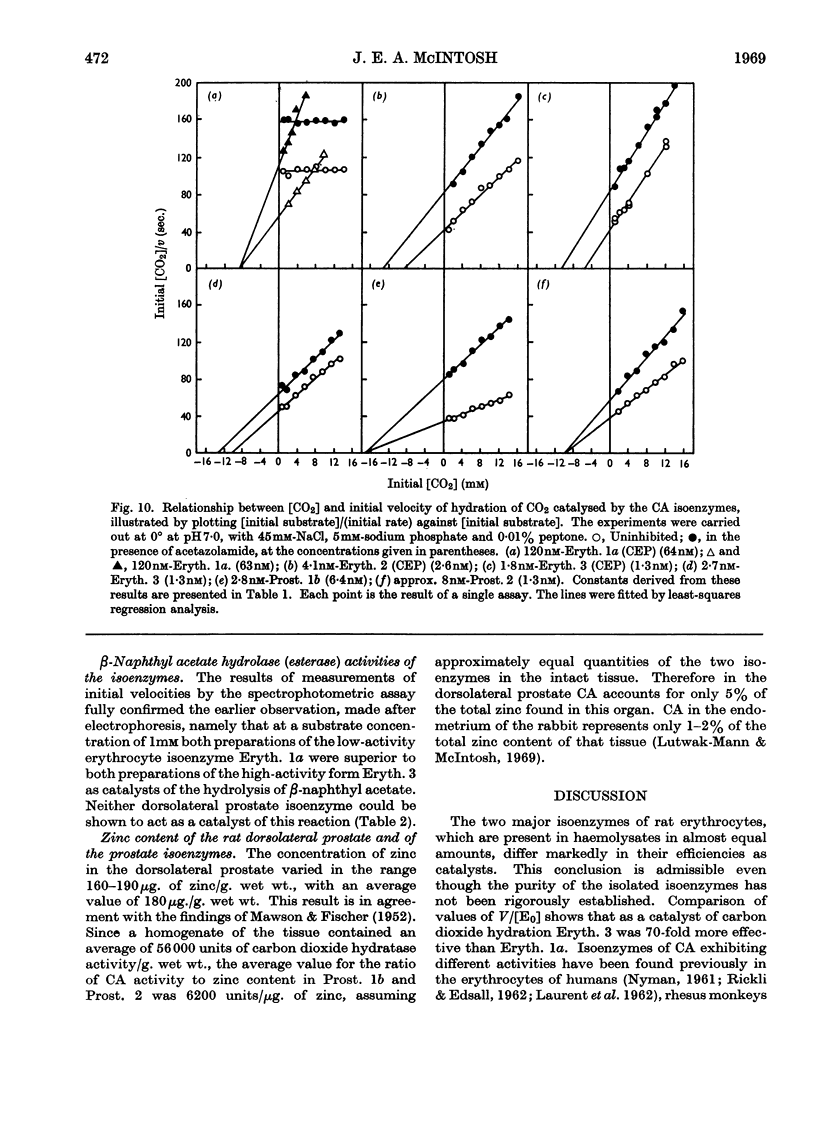
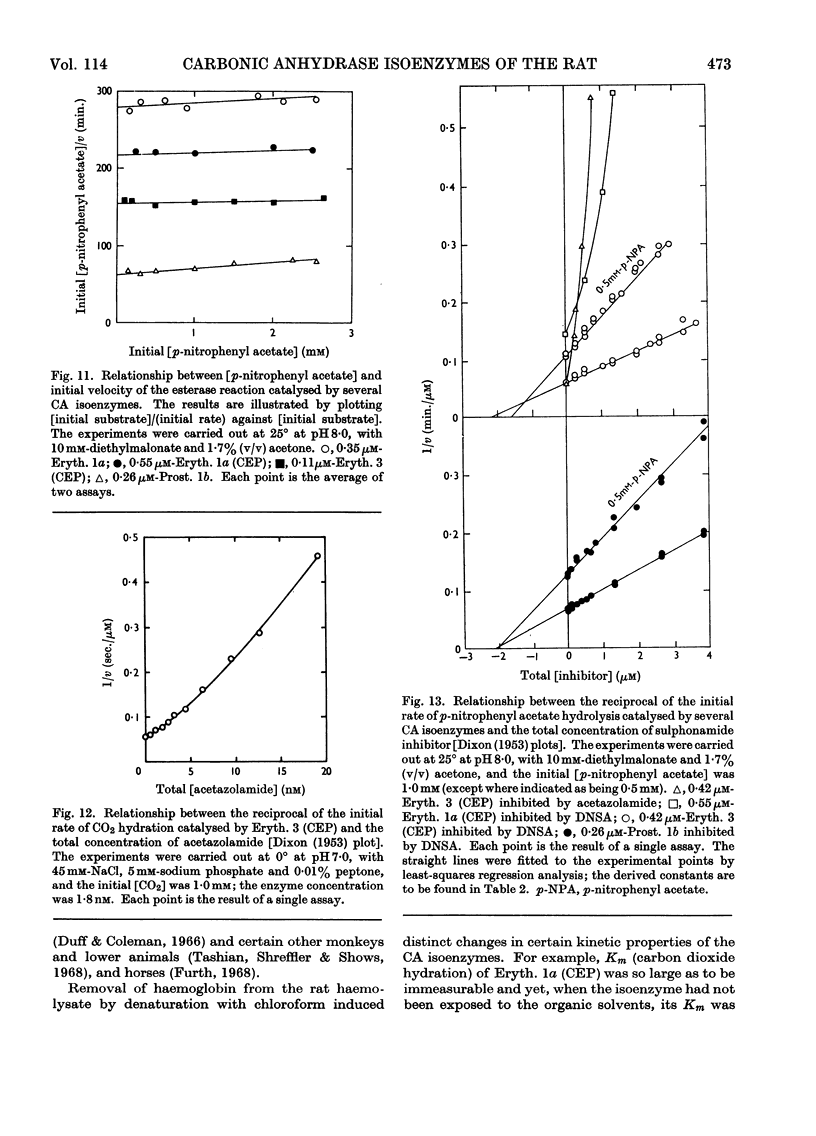
1. Three forms of the zinc-containing enzyme carbonic anhydrase (EC 4.2.1.1) were isolated from the erythrocytes of the rat and two forms from the dorsolateral prostate of the rat. Several additional minor components were observed but not isolated. Separation of the isoenzymes was achieved by ion-exchange chromatography, polyacrylamide-gel electrophoresis and isoelectric focusing. 2. The general properties of the isolated isoenzymes, their molecular weights and their contents of zinc were closely similar. As catalysts of the hydration of carbon dioxide, however, they were distinctly different. The two most abundant isoenzymes of the erythrocytes, which were found in equal proportions, differed 70-fold in specific activity, whereas the isoenzymes of the dorsolateral prostate were similar to one another and resembled the high-activity component of the erythrocytes. The inhibition of the latter by acetazolamide (5-acetamido-1-thia-3,4-diazole-2-sulphonamide) was mainly competitive, whereas in identical conditions the low-activity erythrocyte component and the dorsolateral prostate isoenzymes were non-competitively inhibited. 3. The use of chloroform–ethanol to remove haemoglobin from the rat haemolysate was found (a) to bring about changes in the kinetic properties of the soluble isoenzymes and (b) to cause the appearance of an additional isoenzyme. 4. The actions were compared of the inhibitors acetazolamide, 1,1-dimethylaminonaphthalene-5-sulphonamide and ethoxzolamide (6-ethoxybenzothiazole-2-sulphonamide) on the hydrolysis of p-nitrophenyl acetate catalysed by the isoenzymes. 5. The low-activity erythrocyte isoenzyme was an efficient catalyst of the hydrolysis of β-naphthyl acetate whereas the high-activity forms were much less active towards this ester. Neither of the isoenzymes present in the dorsolateral prostate catalysed this reaction. 6. Carbonic anhydrase in the rat dorsolateral prostate accounts for no more than 5% of the unusually high content of zinc in this organ.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong J. M., Myers D. V., Verpoorte J. A., Edsall J. T. Purification and properties of human erythrocyte carbonic anhydrases. J Biol Chem. 1966 Nov 10;241(21):5137–5149. [PubMed] [Google Scholar]
- BERGMANN F., RIMON S., SEGAL R. Effect of pH on the activity of eel esterase towards different substrates. Biochem J. 1958 Mar;68(3):493–499. doi: 10.1042/bj0680493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R. F., Kernohan J. C. Combination of bovine carbonic anhydrase with a fluorescent sulfonamide. J Biol Chem. 1967 Dec 25;242(24):5813–5823. [PubMed] [Google Scholar]
- Chrambach A., Reisfeld R. A., Wyckoff M., Zaccari J. A procedure for rapid and sensitive staining of protein fractionated by polyacrylamide gel electrophoresis. Anal Biochem. 1967 Jul;20(1):150–154. doi: 10.1016/0003-2697(67)90272-2. [DOI] [PubMed] [Google Scholar]
- DATTA P. K., SHEPARD T. H., 2nd Intracellular localization of carbonic anhydrase in rat liver and kidney tissues. Arch Biochem Biophys. 1959 Mar;81(1):124–129. doi: 10.1016/0003-9861(59)90182-1. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff T. A., Coleman J. E. Macaca mulata carbonic anhydrase. Crystallization and physicochemical and enzymatic properties of two isozymes. Biochemistry. 1966 Jun;5(6):2009–2019. doi: 10.1021/bi00870a032. [DOI] [PubMed] [Google Scholar]
- Edsall J. T. Multiple molecular forms of carbonic anhydrase in erythrocytes. Ann N Y Acad Sci. 1968 Jun 14;151(1):41–63. doi: 10.1111/j.1749-6632.1968.tb11877.x. [DOI] [PubMed] [Google Scholar]
- Furth A. J. Purification and properties of horse erythrocyte carbonic anhydrases. J Biol Chem. 1968 Sep 25;243(18):4832–4841. [PubMed] [Google Scholar]
- GIBBONS B. H., EDSALL J. T. KINETIC STUDIES OF HUMAN CARBONIC ANHYDRASES B AND C. J Biol Chem. 1964 Aug;239:2539–2544. [PubMed] [Google Scholar]
- GUNN S. A., GOULD T. C., GINORI S. S., MORSE J. G. Selective uptake of Zn65 by dorsolateral prostate of rat. Proc Soc Exp Biol Med. 1955 Apr;88(4):556–558. doi: 10.3181/00379727-88-21649. [DOI] [PubMed] [Google Scholar]
- Humphrey G. F., Mann T. Studies on the metabolism of semen. 5. Citric acid in semen. Biochem J. 1949;44(1):97–105. [PMC free article] [PubMed] [Google Scholar]
- KARLER R., WOODBURY D. M. Intracellular distribution of carbonic anhydrase. Biochem J. 1960 Jun;75:538–543. doi: 10.1042/bj0750538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar A. B., Chowdhury A. R. The distribution of zinc in the subcellular fractions of the Rhesus monkey and rat prostate. J Urol. 1966 Sep;96(3):370–371. doi: 10.1016/S0022-5347(17)63271-3. [DOI] [PubMed] [Google Scholar]
- LEIBMAN K. C., ALFORD D., BOUDET R. A. Nature of the inhibition of carbonic anhydrase by acetazolamide and benzthiazide. J Pharmacol Exp Ther. 1961 Mar;131:271–274. [PubMed] [Google Scholar]
- LUTWAK-MANN C. Carbonic anhydrase in the female reproductive tract; occurrence, distribution and hormonal dependence. J Endocrinol. 1955 Oct;13(1):26–38. doi: 10.1677/joe.0.0130026. [DOI] [PubMed] [Google Scholar]
- Lindskog S., Thorslund A. On the interaction of bovine cobalt carbonic anhydrase with sulfonamides. Eur J Biochem. 1968 Feb;3(4):453–460. doi: 10.1111/j.1432-1033.1967.tb19552.x. [DOI] [PubMed] [Google Scholar]
- Lutwak-Mann C., McIntosh J. E. Zinc and carbonic anhydrase in the rabbit uterus. Nature. 1969 Mar 22;221(5186):1111–1114. doi: 10.1038/2211111a0. [DOI] [PubMed] [Google Scholar]
- McIntosh J. E. Assay of carbonic anhydrase by titration at constant pH. Biochem J. 1968 Sep;109(2):203–207. doi: 10.1042/bj1090203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh J., Lutwak-Mann C. Carbonic anhydrase isoenzymes in certain tissues of the male anf female reproductive tract and red blood cells. J Reprod Fertil. 1967 Oct;14(2):344–346. doi: 10.1530/jrf.0.0140344. [DOI] [PubMed] [Google Scholar]
- Pocker Y., Dickerson D. G. The catalytic versatility of erythrocyte carbonic anhydrase. V. Kinetic studies of enzyme-catalyzed hydrations of aliphatic aldehydes. Biochemistry. 1968 May;7(5):1995–2004. doi: 10.1021/bi00845a050. [DOI] [PubMed] [Google Scholar]
- Pocker Y., Stone J. T. The catalytic versatility of erythrocyte carbonic anhydrase. The enzyme-catalyzed hydrolysis of rho-nitrophenyl acetate. J Am Chem Soc. 1965 Dec 5;87(23):5497–5498. doi: 10.1021/ja00951a049. [DOI] [PubMed] [Google Scholar]
- Pocker Y., Stone J. T. The catalytic versatility of erythrocyte carbonic anhydrase. VI. Kinetic studies of noncompetitive inhibition of enzyme-catalyzed hydrolysis of p-nitrophenyl acetate. Biochemistry. 1968 Aug;7(8):2936–2945. doi: 10.1021/bi00848a034. [DOI] [PubMed] [Google Scholar]
- Pocker Y., Stone J. T. The catalytic versatility of erythrocyte carbonic anhydrase. VII. Kinetic studies of esterase activity and competitive inhibition by substrate analogs. Biochemistry. 1968 Sep;7(9):3021–3031. doi: 10.1021/bi00849a001. [DOI] [PubMed] [Google Scholar]
- Pocker Y., Storm D. R. The catalytic versatility of erythrocyte carbonic anhydrase. IV. Kinetic studies of enzyme-catalyzed hydrolyses of para-nitrophenly esters. Biochemistry. 1968 Mar;7(3):1202–1214. doi: 10.1021/bi00843a042. [DOI] [PubMed] [Google Scholar]
- RICKLI E. E., GHAZANFAR S. A., GIBBONS B. H., EDSALL J. T. CARBONIC ANHYDRASES FROM HUMAN ERYTHROCYTES. PREPARATION AND PROPERTIES OF TWO ENZYMES. J Biol Chem. 1964 Apr;239:1065–1078. [PubMed] [Google Scholar]
- RIDDIFORD L. M. HYDROGEN ION EQUILIBRIA OF HUMAN CARBONIC ANHYDRASE B. J Biol Chem. 1964 Apr;239:1079–1086. [PubMed] [Google Scholar]
- STERNBERG S. S., CRONIN A., PHILIPS F. S. HISTOCHEMICAL DEMONSTRATION OF ZINC IN THE DORSOLATERAL PROSTATE OF THE RAT: STUDIES WITH OXINE AND DITHIZONE. Am J Pathol. 1965 Aug;47:325–337. [PMC free article] [PubMed] [Google Scholar]
- TASHIAN R. E. GENETIC VARIATION AND EVOLUTION OF THE CARBOXYLIC ESTERASES AND CARBONIC ANHYDRASES OF PRIMATE ERYTHROCYTES. Am J Hum Genet. 1965 May;17:257–272. [PMC free article] [PubMed] [Google Scholar]
- TASHIAN R. E., SHAW M. W. Inheritance of an erythrocyte acetylesterase variant in man. Am J Hum Genet. 1962 Sep;14:295–300. [PMC free article] [PubMed] [Google Scholar]
- Tashian R. E., Shreffler D. C., Shows T. B. Genetic and phylogenetic variation in the different molecular forms of mammalian erythrocyte carbonic anhydrases. Ann N Y Acad Sci. 1968 Jun 14;151(1):64–77. doi: 10.1111/j.1749-6632.1968.tb11878.x. [DOI] [PubMed] [Google Scholar]
- Thorslund A., Lindskog S. Studies of the esterase activity and the anion inhibition of bovine zinc and cobalt carbonic anhydrases. Eur J Biochem. 1967 Dec;3(1):117–123. doi: 10.1111/j.1432-1033.1967.tb19504.x. [DOI] [PubMed] [Google Scholar]
- Verpoorte J. A., Mehta S., Edsall J. T. Esterase activities of human carbonic anhydrases B and C. J Biol Chem. 1967 Sep 25;242(18):4221–4229. [PubMed] [Google Scholar]
- WEBER G. Polarization of the fluorescence of macromolecules. II. Fluorescent conjugates of ovalbumin and bovine serum albumin. Biochem J. 1952 May;51(2):155–167. doi: 10.1042/bj0510155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIEME R. J. A PROCEDURE FOR HIGH VOLTAGE ELECTROPHORESIS IN AGAR GEL, WITH A NOTE ON ITS APPLICATION TO ACRYLAMIDE AND STARCH GEL. Ann N Y Acad Sci. 1964 Dec 28;121:366–372. doi: 10.1111/j.1749-6632.1964.tb14209.x. [DOI] [PubMed] [Google Scholar]
- Whitney P. L., Fölsch G., Nyman P. O., Malmström B. G. Inhibition of human erythrocyte carbonic anhydrase B by chloroacetyl sulfonamides with labeling of the active site. J Biol Chem. 1967 Sep 25;242(18):4206–4211. [PubMed] [Google Scholar]


