Abstract
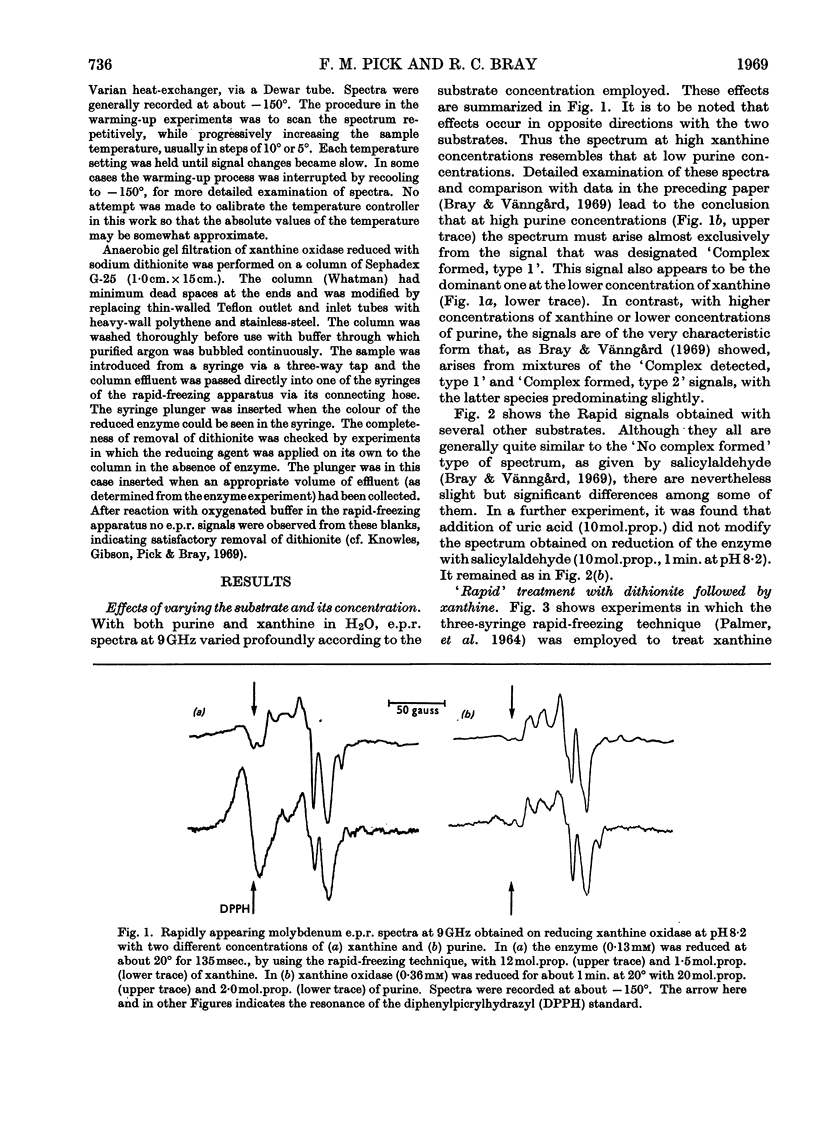
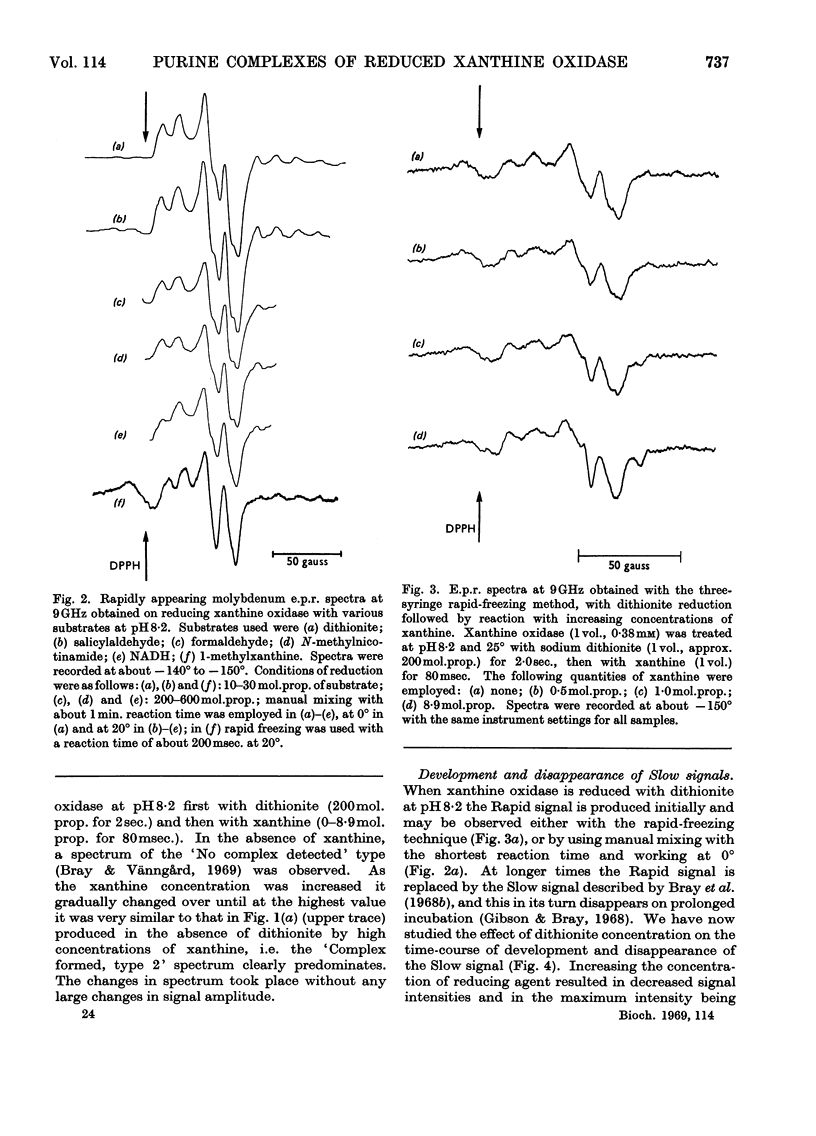
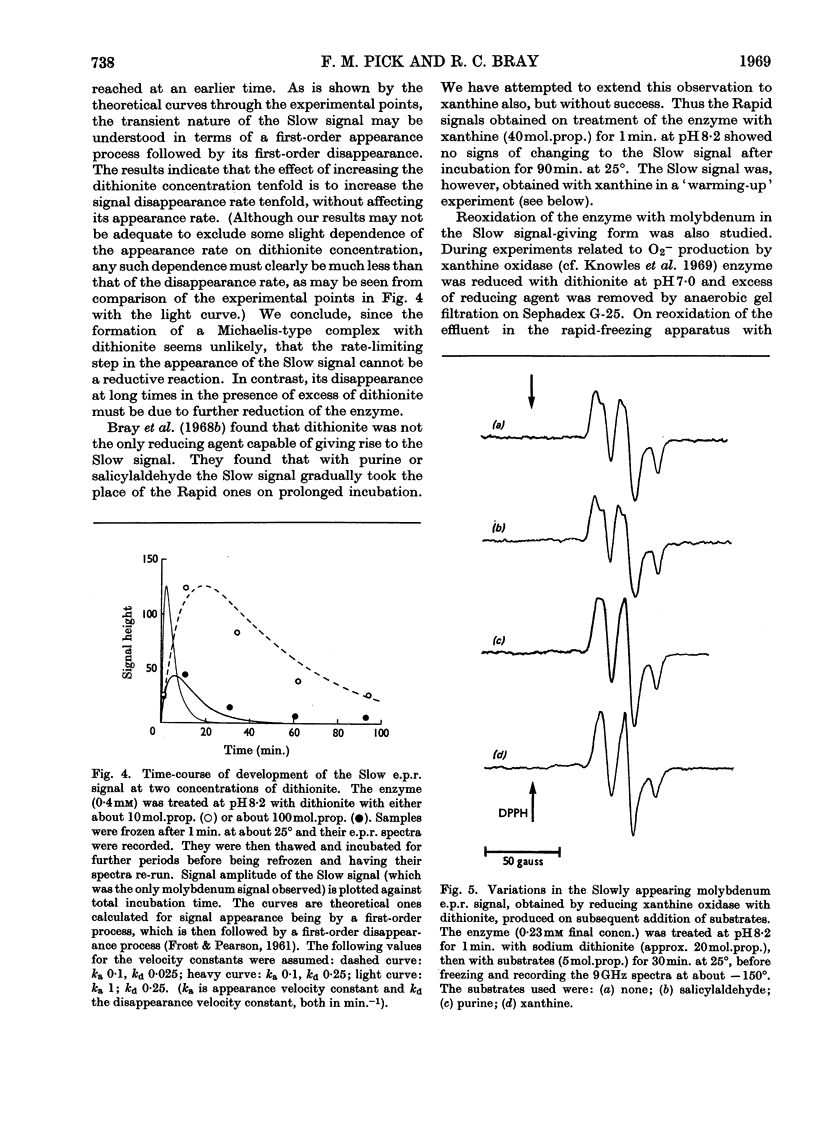
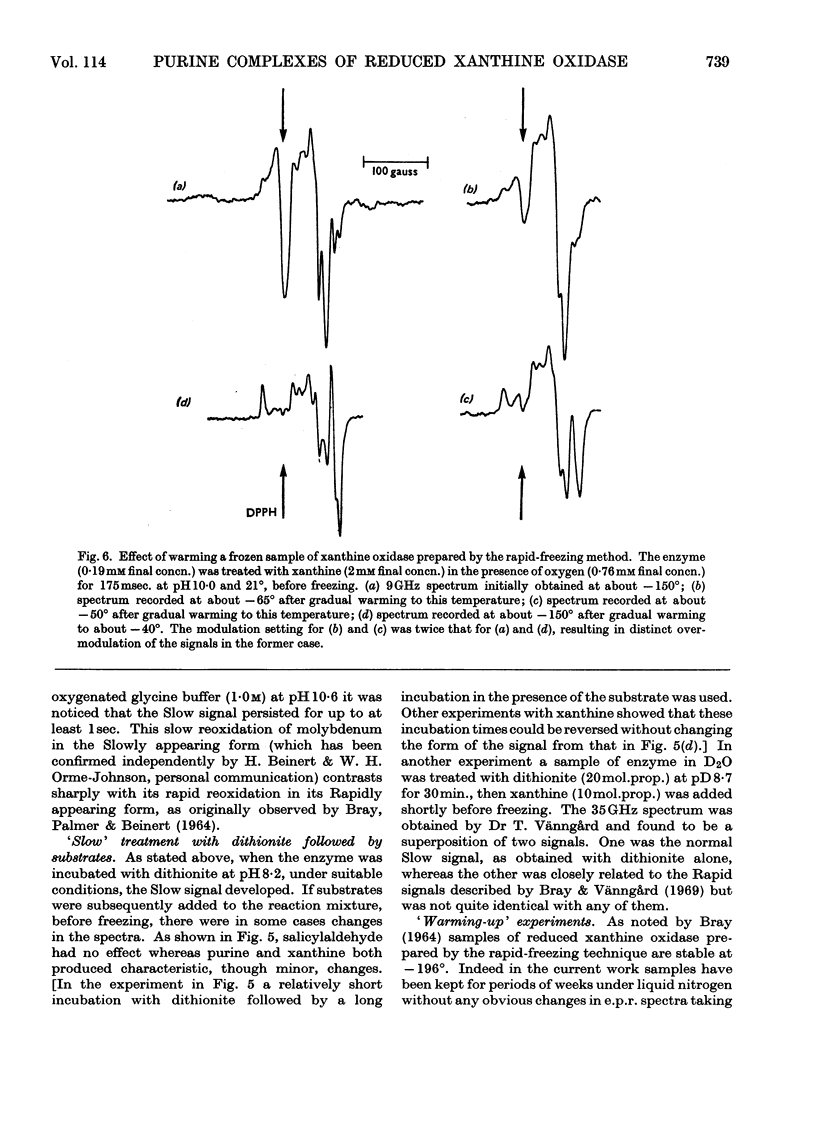
The origin of the Rapid molybdenum electron-paramagnetic-resonance signals, which are obtained on reducing xanthine oxidase with purine or with xanthine, and whose parameters were measured by Bray & Vänngård (1969), was studied. It is concluded that these signals represent complexes of reduced enzyme with substrate molecules. Xanthine forms one complex at high concentrations and a different one at low concentrations. Purine forms a complex indistinguishable from the low-concentration xanthine complex. There are indications that some other substrates also form complexes, but uric acid, a reaction product, does not appear to do so. The possible significance of the complexes in the catalytic cycle of the enzyme is discussed and it is suggested that they represent substrate molecules bound at the reduced active site, waiting their turn to react there, when the enzyme has been reoxidized. Support for this role for the complexes was deduced from experiments in which frozen samples of enzyme–xanthine mixtures, prepared by the rapid-freezing method, were warmed until the signals began to change. Under these conditions an increase in amplitude of the Very Rapid signal took place. Data bearing on the origin of the Slow molybdenum signal are also discussed. This signal disappears only slowly in the presence of oxygen, and its appearance rate is unaffected by change in the concentration of dithionite. It is concluded that, like other signals from the enzyme, it is due to Mov but that a slow change of ligand takes place before it is seen. The Slow species, like the Rapid, seems capable of forming complexes with purines.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRAY R. C., PALMER G., BEINERT H. DIRECT STUDIES ON THE ELECTRON TRANSFER SEQUENCE IN XANTHINE OXIDASE BY ELECTRON PARAMAGNETIC RESONANCE SPECTROSCOPY. II. KINETIC STUDIES EMPLOYING RAPID FREEZING. J Biol Chem. 1964 Aug;239:2667–2676. [PubMed] [Google Scholar]
- BRAY R. C. Sudden freezing as a technique for the study of rapid reactions. Biochem J. 1961 Oct;81:189–193. doi: 10.1042/bj0810189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth V. H. The xanthine oxidase-aldehyde system. Biochem J. 1938 Mar;32(3):503–507. doi: 10.1042/bj0320503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray R. C., Knowles P. F., Pick F. M., Vänngård T. Electron-spin-resonance evidence for interaction of protons with Mo(V) in reduced forms of xanthine oxidase. Biochem J. 1968 Apr;107(4):601–602. doi: 10.1042/bj1070601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray R. C., Vänngård T. "Rapidly appearing" molybdenum electron-paramagnetic-resonance signals from reduced xanthine oxidase. Biochem J. 1969 Oct;114(4):725–734. doi: 10.1042/bj1140725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson J. F., Bray R. C. Electron spin resonance of non-haem iron in xanthine oxidase. Biochim Biophys Acta. 1968 Apr 2;153(3):721–723. doi: 10.1016/0005-2728(68)90201-6. [DOI] [PubMed] [Google Scholar]
- Knowles P. F., Gibson J. F., Pick F. M., Bray R. C. Electron-spin-resonance evidence for enzymic reduction of oxygen to a free radical, the superoxide ion. Biochem J. 1969 Jan;111(1):53–58. doi: 10.1042/bj1110053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey V., Brumby P. E., Komai H. Studies on milk xanthine oxidase. Some spectral and kinetic properties. J Biol Chem. 1969 Apr 10;244(7):1682–1691. [PubMed] [Google Scholar]
- PALMER G., BRAY R. C., BEINERT H. DIRECT STUDIES ON THE ELECTRON TRANSFER SEQUENCE IN XANTHINE OXIDASE BY ELECTRON PARAMAGNETIC RESONANCE SPECTROSCOPY. I. TECHNIQUES AND DESCRIPTION OF SPECTRA. J Biol Chem. 1964 Aug;239:2657–2666. [PubMed] [Google Scholar]
- Rajagopalan K. V., Handler P., Palmer G., Beinert H. Studies of aldehyde oxidase by electron paramagnetic resonance spectroscopy. II. Kinetic studies by rapid freezing. J Biol Chem. 1968 Jul 25;243(14):3797–3806. [PubMed] [Google Scholar]


