Abstract
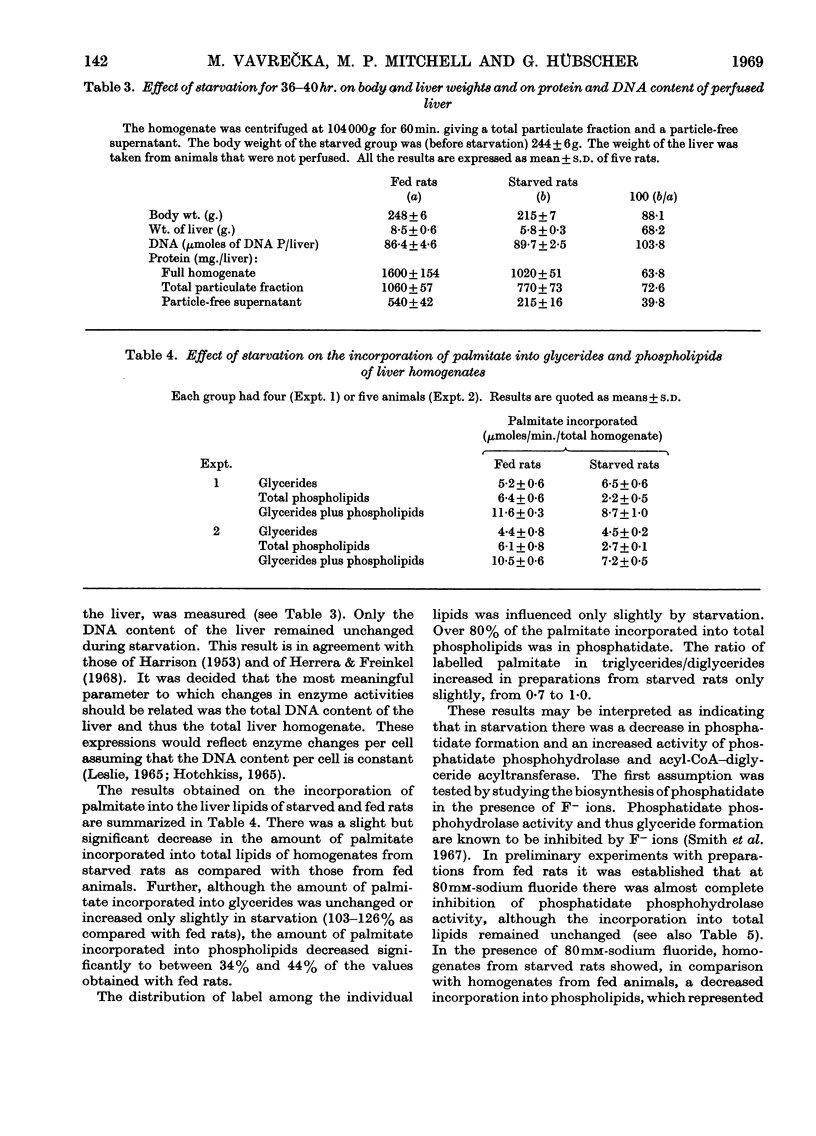
1. Glyceride biosynthesis from glycerol phosphate and [1-14C]palmitate was studied in liver homogenates of rats that were fed ad libitum or starved for 36–40hr. The changes in enzyme activity were related to total DNA content or total liver homogenate as these were found to be equivalent and to be the most meaningful parameters. 2. In liver homogenates from fed rats, labelled palmitate was incorporated mainly into phosphatidate (58% of the total incorporation into lipids), diglycerides (25%) and triglycerides (16%), whereas monoglycerides, cholesterol esters and phospholipids other than phosphatidate were labelled only to a small extent. Addition of particle-free supernatant to full homogenates increased the total incorporation of palmitate by 45% and the pattern of incorporation altered to 53% incorporated into triglycerides, 24% into diglycerides and 17% into phosphatidate. This result suggested that, in liver homogenates, phosphatidate phosphohydrolase (EC 3.1.3.4) may be rate-limiting in the biosynthesis of glycerides via the glycerol phosphate pathway. 3. Upon starvation, the amount of palmitate incorporated per liver into total phospholipids plus glycerides was decreased to between 68% and 75% of that observed with fed animals. In homogenates from fed animals 41–44% of the labelled phospholipids plus glycerides was in glycerides; this value increased to between 63% and 75% with starved rats. Of the palmitate incorporated into total phospholipids, between 85% and 86% was found in phosphatidate, independent of the nutritional state of the animal. The ratio of palmitate incorporated into triglycerides/diglycerides rose from 0·7, obtained with fed rats, to 1·0 with starved animals. 4. These results indicate that starvation caused a decrease in the activity (per total liver) of acyl-CoA–glycerol phosphate acyltransferase(s) (EC 2.3.1.15) and an increase in the activity of acyl-CoA–diglyceride acyltransferase (EC 2.3.1.20). The largest change, however, seemed to be related to the increased activity of the phosphatidate phosphohydrolase in the particle-free supernatant. 5. The latter enzyme was assayed in the particle-free supernatant with membrane-bound phosphatidate as substrate. In starvation, the activity per total liver was increased to between 130% and 190% and the specific activity to between 180% and 320% of the values for fed rats.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLMANN D. W., HUBBARD D. D., GIBSON D. M. FATTY ACID SYNTHESIS DURING FAT-FREE REFEEDING OF STARVED RATS. J Lipid Res. 1965 Jan;6:63–74. [PubMed] [Google Scholar]
- AYDIN A., SOKAL J. E. UPTAKE OF PLASMA FREE FATTY ACIDS BY THE ISOLATED RAT LIVER: EFFECT OF GLUCAGON. Am J Physiol. 1963 Oct;205:667–670. doi: 10.1152/ajplegacy.1963.205.4.667. [DOI] [PubMed] [Google Scholar]
- BRAGDON J. H., GORDON R. S., Jr Tissue distribution of C14 after the intravenous injection of labeled chylomicrons and unesterified fatty acids in the rat. J Clin Invest. 1958 Apr;37(4):574–578. doi: 10.1172/JCI103640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker N., Schotz M. C. Quantitative aspects of free fatty acid metabolism in the fasted rat. J Lipid Res. 1967 Nov;8(6):646–660. [PubMed] [Google Scholar]
- CLARK B., HUBSCHER G. Biosynthesis of glycerides in subcellular fractions of intestinal mucosa. Biochim Biophys Acta. 1961 Jan 29;46:479–494. doi: 10.1016/0006-3002(61)90579-0. [DOI] [PubMed] [Google Scholar]
- Denton R. M., Halperin M. L. The control of fatty acid and triglyceride synthesis in rat epididymal adipose tissue. Roles of coenzyme A derivatives, citrate and L-glycerol 3-phosphate. Biochem J. 1968 Nov;110(1):27–38. doi: 10.1042/bj1100027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton R. M., Randle P. J. Measurement of flow of carbon atoms from glucose and glycogen glucose to glyceride glycerol and glycerol in rat heart and epididymal adipose tissue. Effects of insulin, adrenaline and alloxan-diabetes. Biochem J. 1967 Aug;104(2):423–434. doi: 10.1042/bj1040423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FINE M. B., WILLIAMS R. H. Effect of fasting, epinephrine and glucose and insulin on hepatic uptake of nonesterified fatty acids. Am J Physiol. 1960 Sep;199:403–406. doi: 10.1152/ajplegacy.1960.199.3.403. [DOI] [PubMed] [Google Scholar]
- FREDRICKSON D. S., GORDON R. S., Jr Transport of fatty acids. Physiol Rev. 1958 Oct;38(4):585–630. doi: 10.1152/physrev.1958.38.4.585. [DOI] [PubMed] [Google Scholar]
- Fallon H. J., Kemp E. L. Effects of diet on hepatic triglyceride synthesis. J Clin Invest. 1968 Apr;47(4):712–719. doi: 10.1172/JCI105766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farstad M. In vivo and in vitro studies of the regulation of palmityl-CoA synthetase activity in rat liver. Acta Physiol Scand. 1968 Dec;74(4):568–576. doi: 10.1111/j.1748-1716.1968.tb04268.x. [DOI] [PubMed] [Google Scholar]
- Foster D. W. Studies in the ketosis of fasting. J Clin Invest. 1967 Aug;46(8):1283–1296. doi: 10.1172/JCI105621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOERANSSON G., OLIVECRONA T. THE METABOLISM OF FATTY ACIDS IN THE RAT. I. PALMITIC ACID. Acta Physiol Scand. 1964 Nov;62:224–239. doi: 10.1111/j.1748-1716.1964.tb03970.x. [DOI] [PubMed] [Google Scholar]
- HANAHAN D. J., DITTMER J. C., WARASHINA E. A column chromatographic separation of classes of phospholipides. J Biol Chem. 1957 Oct;228(2):685–700. [PubMed] [Google Scholar]
- HARRISON M. F. Effect of starvation on the composition of the liver cell. Biochem J. 1953 Sep;55(2):204–211. doi: 10.1042/bj0550204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILLYARD L. A., CORNELIUS C. E., CHAIKOFF I. L. Removal by the isolated rat liver of palmitate-1-C14 bound to albumin and of palmitate-1-C14 and cholesterol-4-C14 in chylomicrons from perfusion fluid. J Biol Chem. 1959 Sep;234:2240–2245. [PubMed] [Google Scholar]
- HUBSCHER G., CLARK B. Metabolism of phospholipids. II. Isolation and properties of phosphatidic acid from mammalian liver. Biochim Biophys Acta. 1960 Jun 17;41:45–54. doi: 10.1016/0006-3002(60)90367-x. [DOI] [PubMed] [Google Scholar]
- Hajra A. K. Biosynthesis of acyl dihydroxyacetone phosphate in guinea pig liver mitochondria. J Biol Chem. 1968 Jun 25;243(12):3458–3465. [PubMed] [Google Scholar]
- Herrera E., Freinkel N. Interrelationships between liver composition, plasma glucose and ketones, and hepatic acetyl-CoA and citric acid during prolonged starvation in the male rat. Biochim Biophys Acta. 1968 Dec 23;170(2):244–253. doi: 10.1016/0304-4165(68)90004-4. [DOI] [PubMed] [Google Scholar]
- Hübscher G., Brindley D. N., Smith M. E., Sedgwick B. Stimulation of biosynthesis of glyceride. Nature. 1967 Nov 4;216(5114):449–453. doi: 10.1038/216449a0. [DOI] [PubMed] [Google Scholar]
- Hübscher G., West G. R., Brindley D. N. Studies on the fractionation of mucosal homogenates from the small intestine. Biochem J. 1965 Dec;97(3):629–642. doi: 10.1042/bj0970629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston J. M., Rao G. A., Lowe P. A., Schwarz B. E. The nature of the stimulatory role of the supernatant fraction on triglyceride synthesis by the alpha-Glycerophosphate pathway. Lipids. 1967 Jan;2(1):14–20. doi: 10.1007/BF02531994. [DOI] [PubMed] [Google Scholar]
- KORNBERG A., PRICER W. E., Jr Enzymatic esterification of alpha-glycerophosphate by long chain fatty acids. J Biol Chem. 1953 Sep;204(1):345–357. [PubMed] [Google Scholar]
- LAURELL S. Recycling of intravenously injected palmitic acid-1-C14 as esterified fatty acid in the plasma of rats and turnover rate of plasma triglycerides. Acta Physiol Scand. 1959 Nov 15;47:218–232. doi: 10.1111/j.1748-1716.1960.tb00072.x. [DOI] [PubMed] [Google Scholar]
- MAYES P. A. A calorie deficiency hypothesis of ketogenesis. Metabolism. 1962 Aug;11:781–799. [PubMed] [Google Scholar]
- Mayes P. A., Felts J. M. Regulation of fat metabolism of the liver. Nature. 1967 Aug 12;215(5102):716–718. doi: 10.1038/215716a0. [DOI] [PubMed] [Google Scholar]
- Newsholme E. A., Gevers W. Control of glycolysis and gluconeogenesis in liver and kidney cortex. Vitam Horm. 1967;25:1–87. doi: 10.1016/s0083-6729(08)60033-3. [DOI] [PubMed] [Google Scholar]
- Pande S. V., Mead J. F. Long chain fatty acid activation in subcellular preparations from rat liver. J Biol Chem. 1968 Jan 25;243(2):352–361. [PubMed] [Google Scholar]
- Rao G. A., Wiegand R. D., Reiser R. Soluble acyltransferases in rat liver. Biochim Biophys Acta. 1969 Mar 4;176(2):423–425. doi: 10.1016/0005-2760(69)90203-3. [DOI] [PubMed] [Google Scholar]
- Rubenstein B., Rubinstein D. The effect of fasting on esterification of palmitate by rat liver in vitro. Can J Biochem. 1966 Jan;44(1):129–140. doi: 10.1139/o66-014. [DOI] [PubMed] [Google Scholar]
- SCHIMKE R. T. Adaptive characteristics of urea cycle enzymes in the rat. J Biol Chem. 1962 Feb;237:459–468. [PubMed] [Google Scholar]
- Schotz M. C., Olivecrona T. The effect of anesthesia on the fate of injected free fatty acid. Biochim Biophys Acta. 1966 Aug 3;125(1):174–175. doi: 10.1016/0005-2760(66)90155-x. [DOI] [PubMed] [Google Scholar]
- Shephard E. H., Hübscher G. Phosphatidate biosynthesis in mitochondrial subfractions of rat liver. Biochem J. 1969 Jun;113(2):429–440. doi: 10.1042/bj1130429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. E., Hübscher G. The biosynthesis of glycerides by mitochondria from rat liver. The requirement for a soluble protein. Biochem J. 1966 Nov;101(2):308–316. doi: 10.1042/bj1010308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. E., Sedgwick B., Brindley D. N., Hübscer G. The role of phosphatidate phosphohydrolase in glyceride biosynthesis. Eur J Biochem. 1967 Dec;3(1):70–77. doi: 10.1111/j.1432-1033.1967.tb19499.x. [DOI] [PubMed] [Google Scholar]
- TZUR R., TAL E., SHAPIRO B. ALPHA-GLYCEROPHOSPHATE AS REGULATORY FACTOR IN FATTY ACID ESTERIFICATION. Biochim Biophys Acta. 1964 Feb 24;84:18–23. doi: 10.1016/0926-6542(64)90096-4. [DOI] [PubMed] [Google Scholar]
- Tubbs P. K., Garland P. B. Variations in tissue contents of coenzyme A thio esters and possible metabolic implications. Biochem J. 1964 Dec;93(3):550–557. doi: 10.1042/bj0930550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEBER G., BANERJEE G., BRONSTEIN S. B. Role of enzymes in homeostasis. III. Selective induction of increases of liver enzymes involved in carbohydrate metabolism. J Biol Chem. 1961 Dec;236:3106–3111. [PubMed] [Google Scholar]
- WILGRAM G. F., KENNEDY E. P. INTRACELLULAR DISTRIBUTION OF SOME ENZYMES CATALYZING REACTIONS IN THE BIOSYNTHESIS OF COMPLEX LIPIDS. J Biol Chem. 1963 Aug;238:2615–2619. [PubMed] [Google Scholar]
- Williams M. A., Tamai K. T., McIntosh D. J. Effects of fasting on liver lipids in rats fed a purified diet. Biochim Biophys Acta. 1967 Feb 14;137(1):187–189. doi: 10.1016/0005-2760(67)90025-2. [DOI] [PubMed] [Google Scholar]


