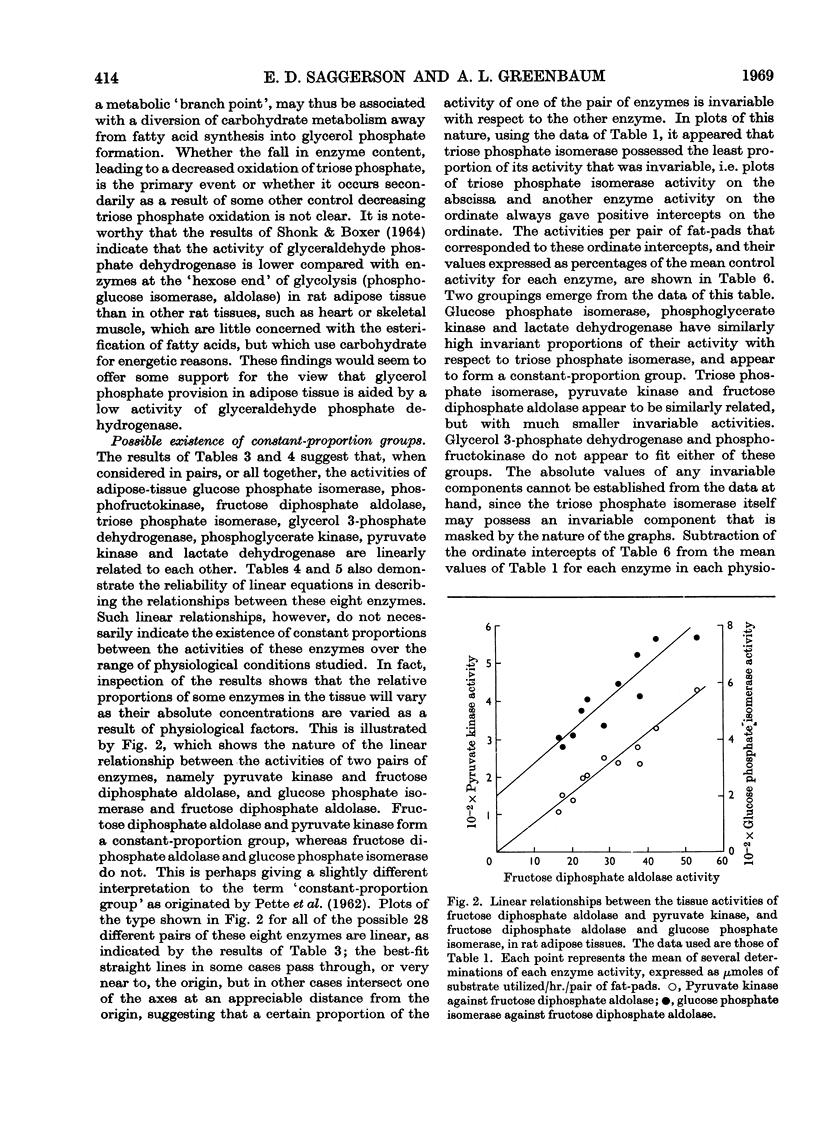
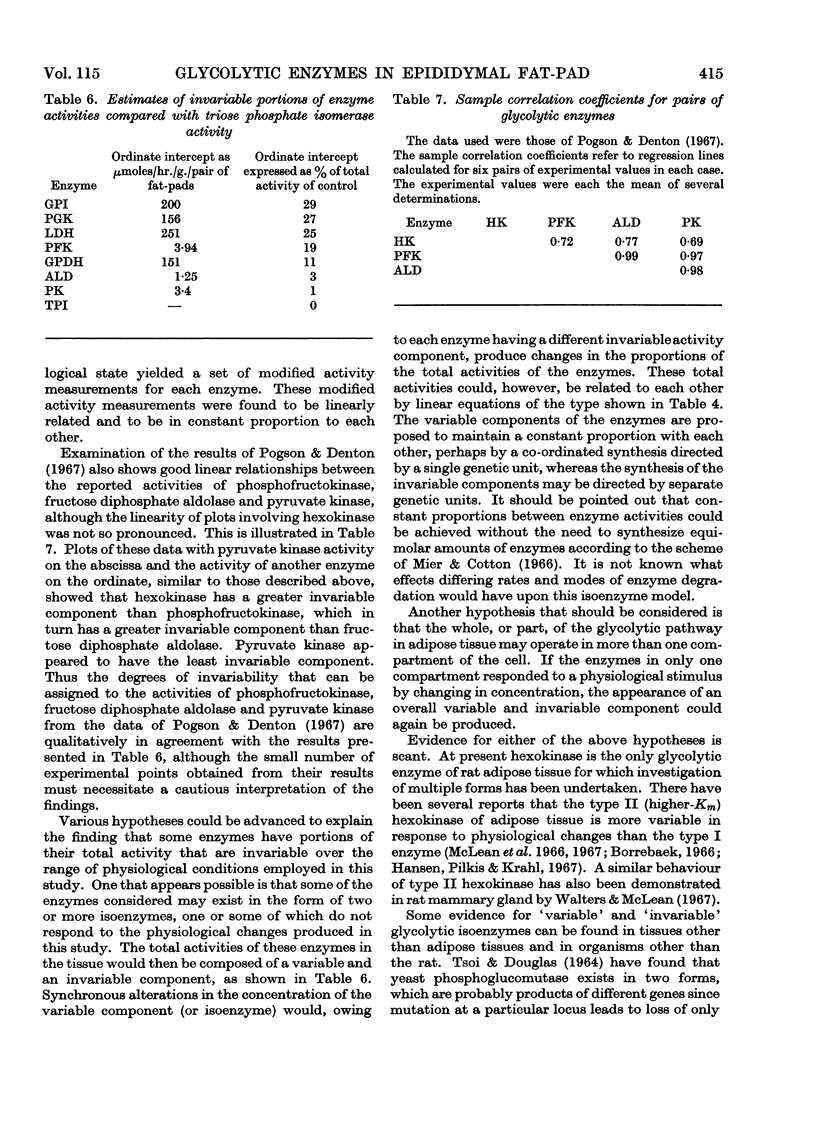
Abstract
1. Measurements were made of the activities of nine glycolytic enzymes in epididymal adipose tissues obtained from rats that had undergone one of the following treatments: starvation; starvation followed by re-feeding with bread or high-fat diet; feeding with fat without preliminary starvation; alloxan-diabetes; alloxan-diabetes followed by insulin therapy. 2. In general, the activities of the glycolytic enzymes of adipose tissue, unlike those of liver, were not greatly affected by the above treatments. 3. The `key' glycolytic enzymes, phosphofructokinase and pyruvate kinase, were generally no more adaptive in response to physiological factors than other glycolytic enzymes such as glucose phosphate isomerase, fructose diphosphate aldolase, triose phosphate isomerase, glycerol 3-phosphate dehydrogenase, phosphoglycerate kinase and lactate dehydrogenase. 4. Adiposetissue pyruvate kinase did not respond to feeding with fat in a manner similar to the liver enzyme. 5. Glyceraldehyde phosphate dehydrogenase had a behaviour pattern unlike the other eight glycolytic enzymes studied in that its activity was depressed by feeding with fat and was not restored to normal by re-feeding with a high-fat diet after starvation. These results are discussed in relation to the requirements of adipose tissue for glycerol phosphate in the esterification of fatty acids. 6. A statistical analysis of the results permitted the writing of linear equations describing the relationships between the activities of eight of the enzymes studied. 7. Evidence is presented for the existence of two constant-proportion groups amongst the enzymes studied, namely (i) glucose phosphate isomerase, phosphoglycerate kinase and lactate dehydrogenase, and (ii) triose phosphate isomerase, fructose diphosphate aldolase and pyruvate kinase. 8. Mechanisms for maintaining the observed relationships between the activities of the enzymes in the tissue are discussed.
Full text
PDF

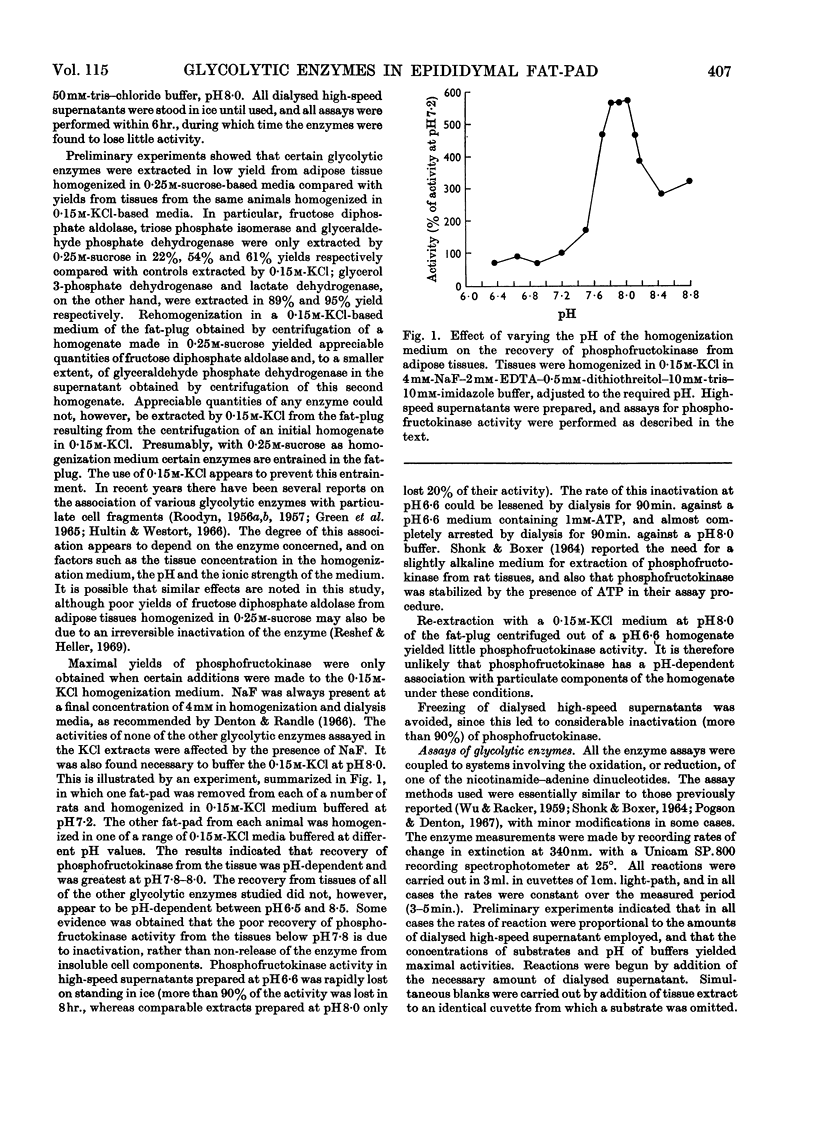

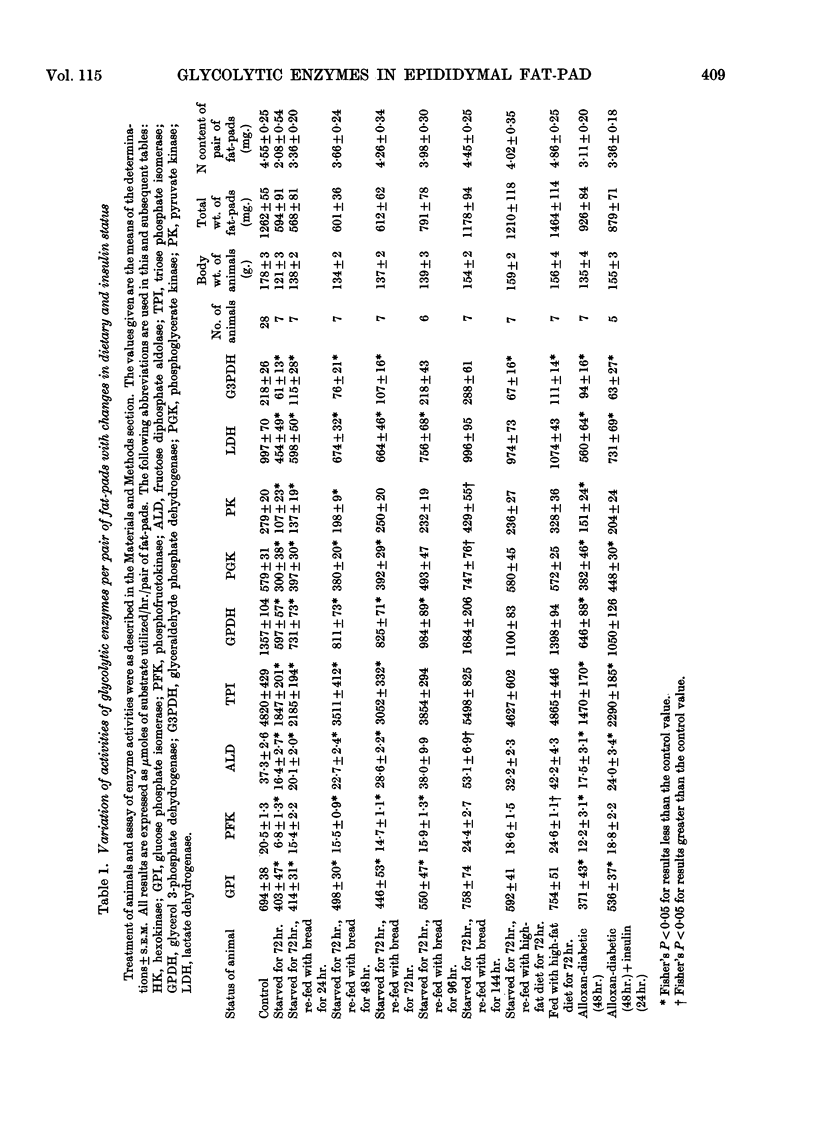
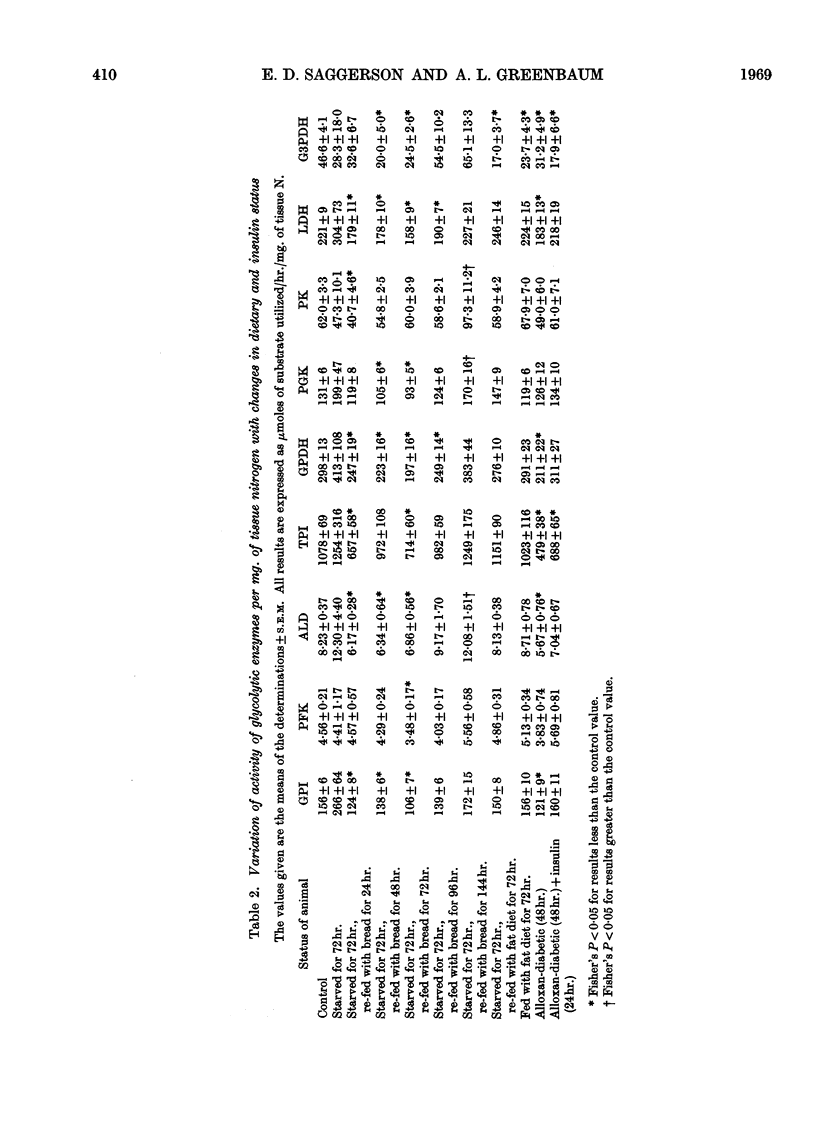

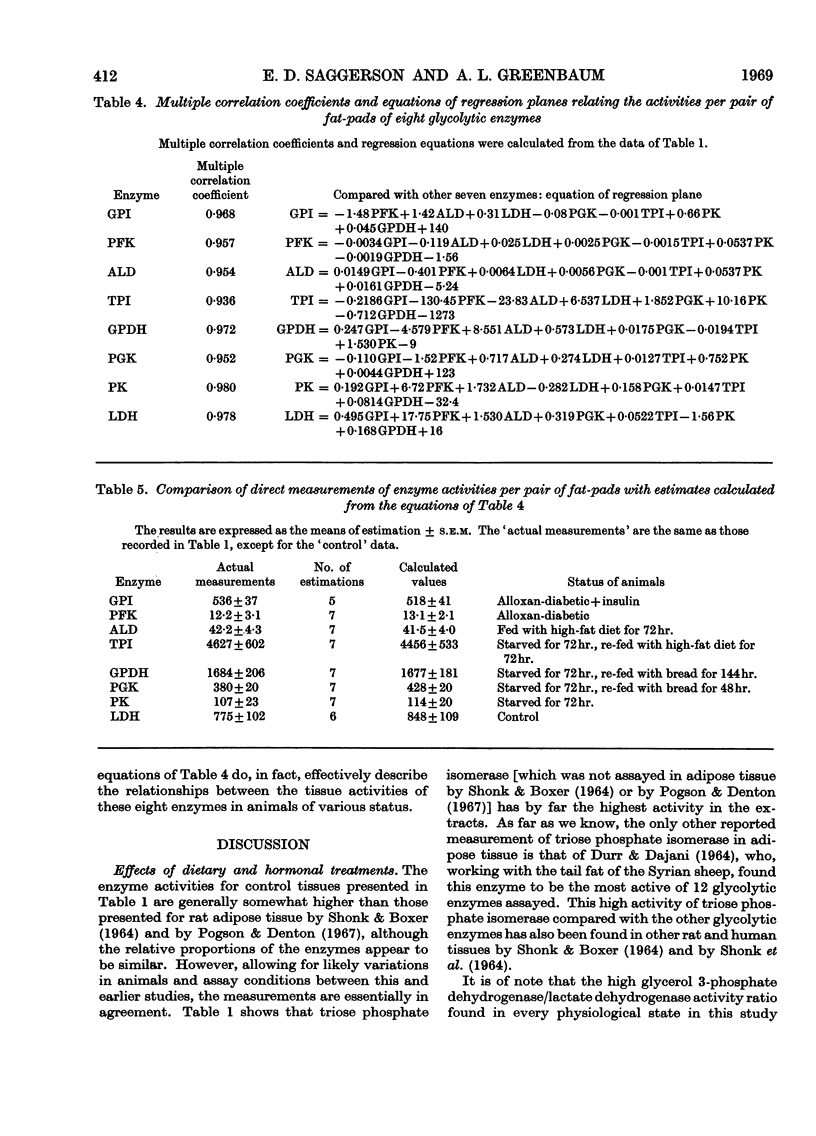



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J., Hollifield G. The effects of starvation and refeeding on hexosemonophosphate shunt enzyme activity and DNA, RNA, and nitrogen content of rat adipose tissue. Metabolism. 1966 Dec;15(12):1098–1103. doi: 10.1016/0026-0495(66)90099-0. [DOI] [PubMed] [Google Scholar]
- Avramov I. A., Repin V. S. Vydelenie vysokoochishchennoi fosfoglitseratkinazy iz skeletnykh mushts krolika. Biokhimiia. 1965 Nov-Dec;30(6):1187–1193. [PubMed] [Google Scholar]
- BAKER N., CHAIKOFF I. L., SCHUSDEK A. Effect of fructose on lipogenesis from lactate and acetate in diabetic liver. J Biol Chem. 1952 Jan;194(1):435–443. [PubMed] [Google Scholar]
- BRUCE H. M., PARKES A. S. Feeding and breeding of laboratory animals; a complete cubed diet for mice and rats. J Hyg (Lond) 1949 Jun;47(2):202–208. doi: 10.1017/s0022172400014479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrebaek B. Increase in epididymal adipose tissue hexokinase activity induced by glucose and insulin. Biochim Biophys Acta. 1966 Oct 17;128(1):211–213. doi: 10.1016/0926-6593(66)90165-2. [DOI] [PubMed] [Google Scholar]
- CROFFORD O. B., RENOLD A. E. GLUCOSE UPTAKE BY INCUBATED RAT EPIDIDYMAL ADIPOSE TISSUE. RATE-LIMITING STEPS AND SITE OF INSULIN ACTION. J Biol Chem. 1965 Jan;240:14–21. [PubMed] [Google Scholar]
- DURR I. F., DAJANI B. OXIDATIVE PATHWAYS IN THE ADIPOSE TISSUE OF THE FAT TAIL OF THE SYRIAN SHEEP. Comp Biochem Physiol. 1964 Nov;13:225–232. doi: 10.1016/0010-406x(64)90119-7. [DOI] [PubMed] [Google Scholar]
- Denton R. M., Randle P. J. Citrate and the regulation of adipose-tissue phosphofructokinase. Biochem J. 1966 Aug;100(2):420–423. doi: 10.1042/bj1000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAWCETT J. K., SCOTT J. E. A rapid and precise method for the determination of urea. J Clin Pathol. 1960 Mar;13:156–159. doi: 10.1136/jcp.13.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FURFINE C. S., VELICK S. F. THE ACYL-ENZYME INTERMEDIATE AND THE KINETIC MECHANISM OF THE GLYCERALDEHYDE 3-PHOSPHATE DEHYDROGENASE REACTION. J Biol Chem. 1965 Feb;240:844–855. [PubMed] [Google Scholar]
- Green D. E., Murer E., Hultin H. O., Richardson S. H., Salmon B., Brierley G. P., Baum H. Association of integrated metabolic pathways with membranes. I. Glycolytic enzymes of the red blood corpuscle and yeast. Arch Biochem Biophys. 1965 Dec;112(3):635–647. doi: 10.1016/0003-9861(65)90107-4. [DOI] [PubMed] [Google Scholar]
- Hansen R., Pilkis S. J., Krahl M. E. Properties of adaptive hexokinase isozymes of the rat. Endocrinology. 1967 Dec;81(6):1397–1404. doi: 10.1210/endo-81-6-1397. [DOI] [PubMed] [Google Scholar]
- Henderson N. S. Isozymes and genetic control of NADP-malate dehydrogenase in mice. Arch Biochem Biophys. 1966 Oct;117(1):28–33. doi: 10.1016/0003-9861(66)90121-4. [DOI] [PubMed] [Google Scholar]
- Hommes F. A. Effect of glucose on the level of glycolytic enzymes in yeast. Arch Biochem Biophys. 1966 Apr;114(1):231–233. doi: 10.1016/0003-9861(66)90325-0. [DOI] [PubMed] [Google Scholar]
- Joshi J. G., Hooper J., Kuwaki T., Sakurada T., Swanson J. R., Handler P. Phosphoglucomutase. V. Multiple forms of phosphoglucomutase. Proc Natl Acad Sci U S A. 1967 May;57(5):1482–1489. doi: 10.1073/pnas.57.5.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzen H. M. The effect of diabetes and insulin in vivo and in vitro on a low Km form of hexokinase from various rat tissues. Biochem Biophys Res Commun. 1966 Aug 23;24(4):531–536. doi: 10.1016/0006-291x(66)90352-4. [DOI] [PubMed] [Google Scholar]
- Kneer P., Ball E. G. Studies on the metabolism of adipose tissue. XXI. An evaluation of the major pathways of pyruvate metabolism. J Biol Chem. 1968 Jun 10;243(11):2863–2870. [PubMed] [Google Scholar]
- Kornacker M. S., Ball E. G. Citrate cleavage in adipose tissue. Proc Natl Acad Sci U S A. 1965 Sep;54(3):899–904. doi: 10.1073/pnas.54.3.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUBOCHINSKY B., ZALTA J. P. Microdosage colorimétrique de l'azote ammoniacal. Bull Soc Chim Biol (Paris) 1954;36(9):1363–1366. [PubMed] [Google Scholar]
- Landau B. R., Sims E. A. On the existence of two separate pools of glucose 6-phosphate in rat diaphragm. J Biol Chem. 1967 Jan 25;242(2):163–172. [PubMed] [Google Scholar]
- McLean P., Brown J., Greenslade K., Brew K. Effect of alloxan-diabetes on the glucose-ATP phosphotransferase activity of adipose tissue. Biochem Biophys Res Commun. 1966 Apr 19;23(2):117–121. doi: 10.1016/0006-291x(66)90514-6. [DOI] [PubMed] [Google Scholar]
- McLean P., Brown J., Walters E., Greenslade K. Effect of alloxan-diabetes on multiple forms of hexokinase in adipose tissue and lung. Biochem J. 1967 Dec;105(3):1301–1305. doi: 10.1042/bj1051301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mier P. D., Cotton D. W. Operon hypothesis: new evidence from the "constant proportion" group of the Embden-Meyerhof pathway. Nature. 1966 Mar 5;209(5027):1022–1023. doi: 10.1038/2091022b0. [DOI] [PubMed] [Google Scholar]
- Moses V., Lonberg-Holm K. K. The study of metabolic compartmentalization. J Theor Biol. 1966 Feb;10(2):336–351. doi: 10.1016/0022-5193(66)90131-7. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y., Noltmann E. A. Multiple forms of yeast phosphoglucose isomerase. I. Resolution of the crystalline enzyme into three isoenzymes. J Biol Chem. 1967 Oct 25;242(20):4782–4788. [PubMed] [Google Scholar]
- PETTE D., LUH W., BUECHER T. A constant-proportion group in the enzyme activity pattern of the Embden-Meyerhof chain. Biochem Biophys Res Commun. 1962 Jun 4;7:419–424. doi: 10.1016/0006-291x(62)90327-3. [DOI] [PubMed] [Google Scholar]
- Pogson C. I. Adipose-tissue pyruvate kinase. Properties and interconversion of two active forms. Biochem J. 1968 Nov;110(1):67–77. doi: 10.1042/bj1100067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogson C. I., Denton R. M. Effect of alloxan diabetes, starvation and refeeding on glycolytic kinase activities in rat epididymal adipose tissue. Nature. 1967 Oct 14;216(5111):156–157. doi: 10.1038/216156a0. [DOI] [PubMed] [Google Scholar]
- RAY W. J., Jr, KOSHLAND D. E., Jr Identification of amino acids involved in phosphoglucomutase action. J Biol Chem. 1962 Aug;237:2493–2505. [PubMed] [Google Scholar]
- ROODYN D. B. The binding of aldolase to isolated nuclei. Biochim Biophys Acta. 1957 Jul;25(1):129–131. doi: 10.1016/0006-3002(57)90427-4. [DOI] [PubMed] [Google Scholar]
- ROODYN D. B. The enzymic properties of rat-liver nuclei. 1. Estimation of the extent to which contaminant material contributes to the activity observed in the nuclear fraction. Biochem J. 1956 Oct;64(2):361–368. doi: 10.1042/bj0640361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reshef L., Heller M. Inactivation and protection of fructose diphosphate aldolase in rat adipose tissue extracts. J Biol Chem. 1969 Feb 25;244(4):766–770. [PubMed] [Google Scholar]
- Reshef L., Niv J., Shapiro B. Effect of propionate on lipogenesis in adipose tissue. J Lipid Res. 1967 Nov;8(6):682–687. [PubMed] [Google Scholar]
- SHONK C. E., BOXER G. E. ENZYME PATTERNS IN HUMAN TISSUES. I. METHODS FOR THE DETERMINATION OF GLYCOLYTIC ENZYMES. Cancer Res. 1964 May;24:709–721. [PubMed] [Google Scholar]
- SHONK C. E., KOVEN B. J., MAJIMA H., BOXER G. E. ENZYME PATTERNS IN HUMAN TISSUES. II. GLYCOLYTIC ENZYME PATTERNS IN NONMALIGNANT HUMAN TISSUES. Cancer Res. 1964 May;24:722–731. [PubMed] [Google Scholar]
- TSOI A., DOUGLAS H. C. THE EFFECT OF MUTATION OF TWO FORMS OF PHOSPHOGLUCOMUTASE IN SACCHAROMYCES. Biochim Biophys Acta. 1964 Dec 23;92:513–520. doi: 10.1016/0926-6569(64)90011-2. [DOI] [PubMed] [Google Scholar]
- Takeda Y., Inoue H., Honjo K., Tanioka H., Daikuhara Y. Dietary response of various key enzymes related to glucose metabolism in normal and diabetic rat liver. Biochim Biophys Acta. 1967 Mar 22;136(2):214–222. doi: 10.1016/0304-4165(67)90066-9. [DOI] [PubMed] [Google Scholar]
- VAUGHAN D. A., HANNON J. P., VAUGHAN L. N. Effects of diet on selected glycolytic enzymes of the rat. Am J Physiol. 1960 Dec;199:1041–1044. doi: 10.1152/ajplegacy.1960.199.6.1041. [DOI] [PubMed] [Google Scholar]
- WISE E. M., Jr, BALL E. G. MALIC ENZYME AND LIPOGENESIS. Proc Natl Acad Sci U S A. 1964 Nov;52:1255–1263. doi: 10.1073/pnas.52.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WU R., RACKER E. Regulatory mechanisms in carbohydrate metabolism. III. Limiting factors in glycolysis of ascites tumor cells. J Biol Chem. 1959 May;234(5):1029–1035. [PubMed] [Google Scholar]
- Walters E., McLean P. Multiple forms of glucose-adenosine triphosphate phosphotransferase in rat mammary gland. Biochem J. 1967 Sep;104(3):778–783. doi: 10.1042/bj1040778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUNG J. W., SHRAGO E., LARDY H. A. METABOLIC CONTROL OF ENZYMES INVOLVED IN LIPOGENESIS AND GLUCONEOGENESIS. Biochemistry. 1964 Nov;3:1687–1692. doi: 10.1021/bi00899a015. [DOI] [PubMed] [Google Scholar]


