Abstract
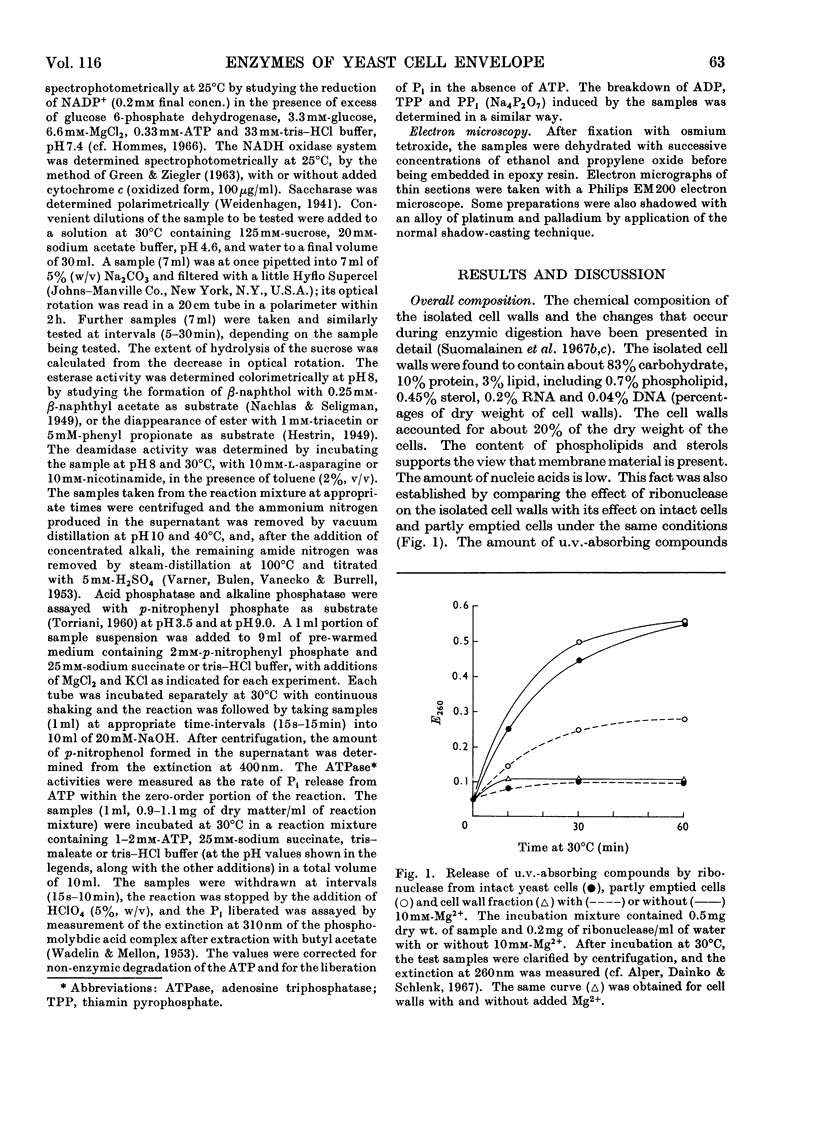
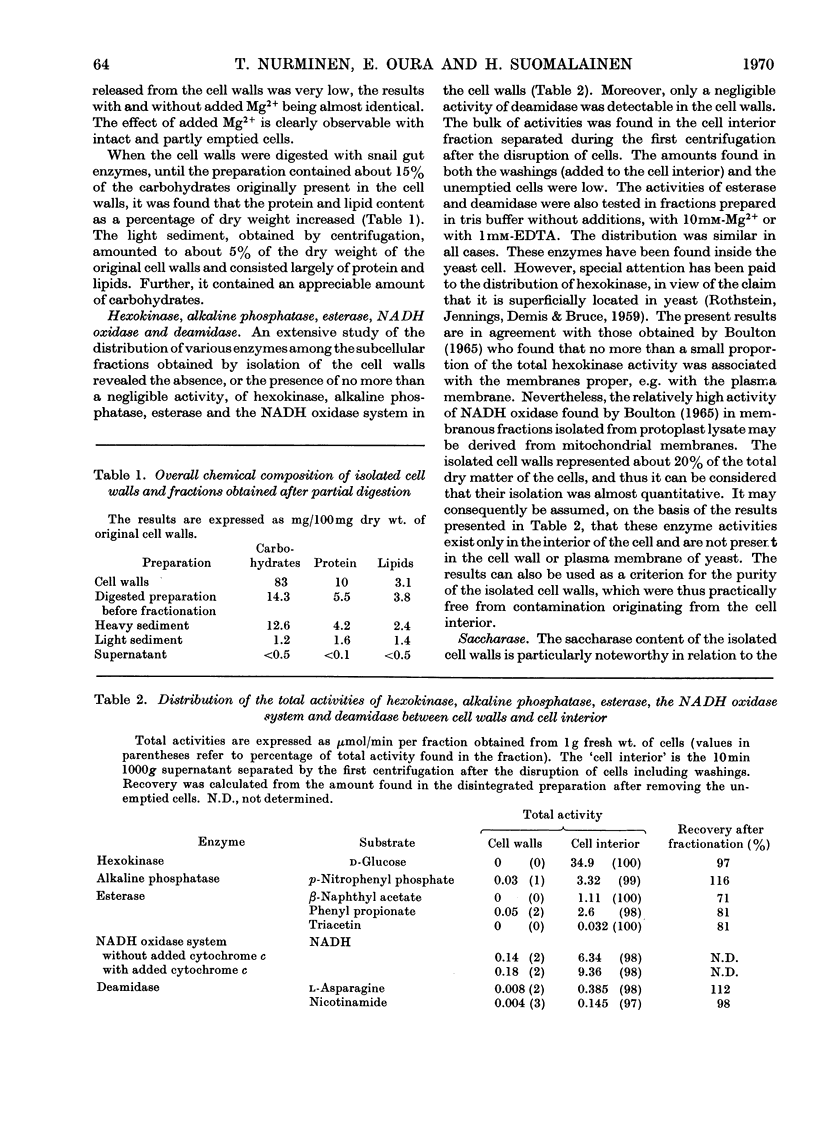
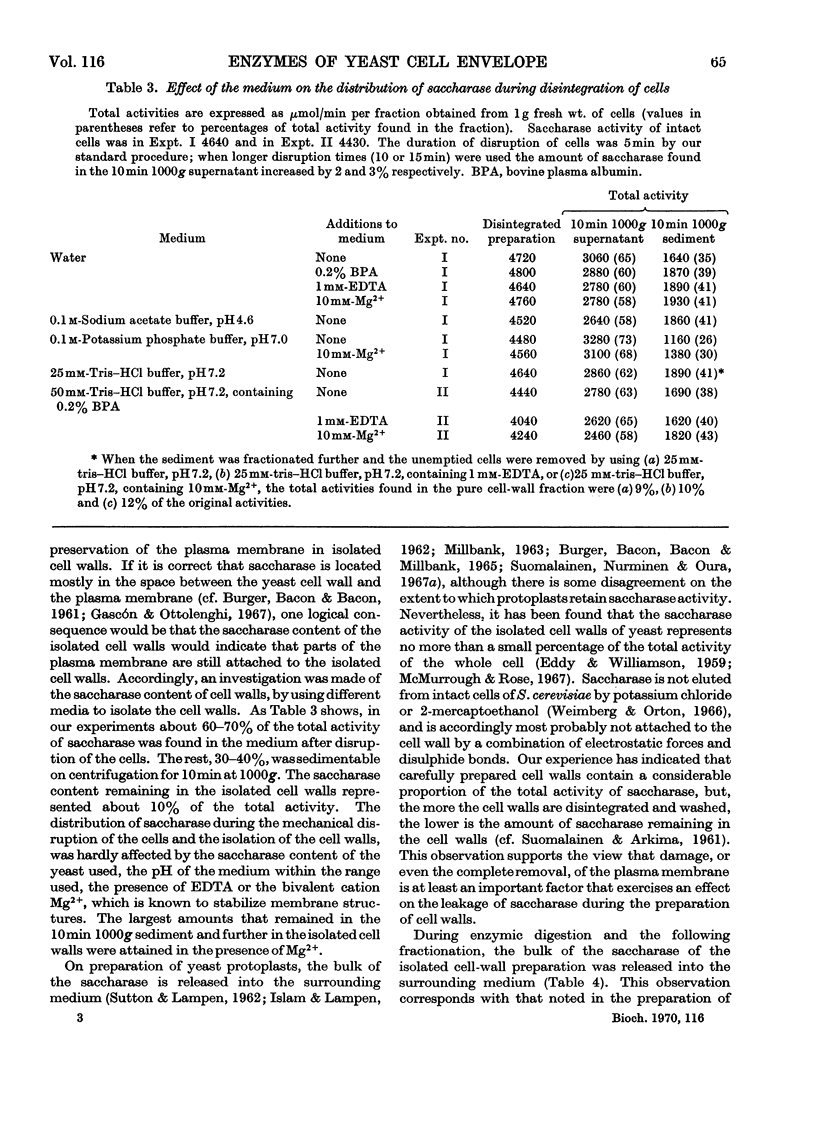
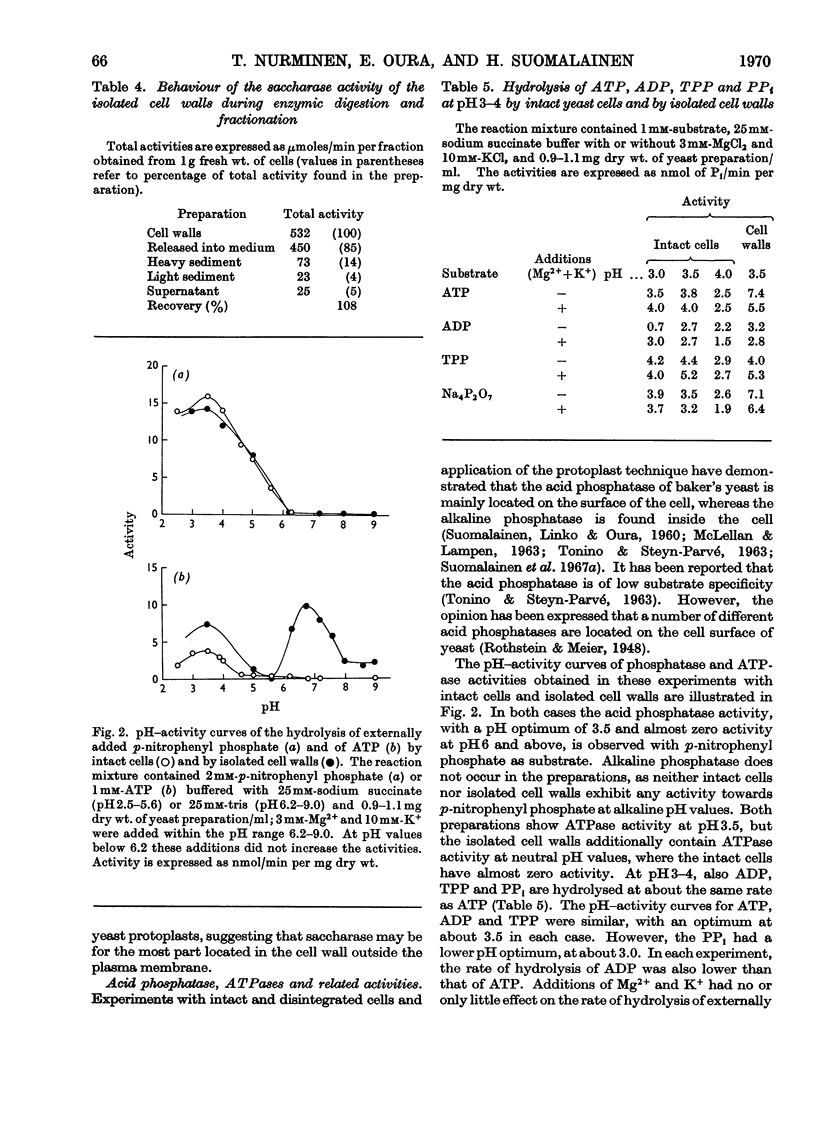
A study was made of the enzyme content of the isolated cell walls and of a plasma-membrane preparation obtained by centrifugation after enzymic digestion of the cell walls of baker's yeast. The isolated cell walls showed no hexokinase, alkaline phosphatase, esterase or NADH oxidase activity. It was concluded that these enzymes exist only in the interior of the cell. Further, only a negligible activity of deamidase was detectable in the cell walls. Noticeable amounts of saccharase, phosphatases hydrolysing p-nitrophenyl phosphate, ATP, ADP, thiamin pyrophosphate and PPi, with optimum activity at pH3–4, and an activity of Mg2+-dependent adenosine triphosphatase at neutral pH, were found in the isolated cell walls. During enzymic digestion, the other activities appearing in the cell walls were mostly released into the medium, but the bulk of the Mg2+-dependent adenosine triphosphatase remained in the plasma-membrane preparation. Accordingly, it may be assumed that the enzymes released into the medium during digestion are located in the cell wall outside the plasma membrane, whereas the Mg2+-dependent adenosine triphosphatase is an enzyme of the plasma membrane. This enzyme differs from the phosphatases with pH optima in the range pH3–4 with regard to location, pH optimum, substrate specificity and different requirement of activators.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. J. The estimation of phosphorus. Biochem J. 1940 Jun;34(6):858–865. doi: 10.1042/bj0340858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alper R. E., Dainko J. L., Schlenk F. Properties of yeast cell ghosts obtained by ribonuclease action. J Bacteriol. 1967 Feb;93(2):759–765. doi: 10.1128/jb.93.2.759-765.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson F. B., Millbank J. W. Protoplast formation and yeast cell-wall structure. The action of the enzymes of the snail, Helix pomatia. Biochem J. 1966 Jun;99(3):682–687. doi: 10.1042/bj0990682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOULTON A. A. SOME OBSERVATIONS ON THE CHEMISTRY AND MORPHOLOGY OF THE MEMBRANES RELEASED FROM YEAST PROTOPLASTS BY OSMOTIC SHOCK. Exp Cell Res. 1965 Feb;37:343–359. doi: 10.1016/0014-4827(65)90183-7. [DOI] [PubMed] [Google Scholar]
- BURGER M., BACON E. E., BACON J. S. Some observations on the form and location of invertase in the yeast cell. Biochem J. 1961 Mar;78:504–511. doi: 10.1042/bj0780504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDDY A. A. [The structure of the yeast cell wall. II. Degradative studies with enzymes]. Proc R Soc Lond B Biol Sci. 1958 Dec 17;149(936):425–440. doi: 10.1098/rspb.1958.0085. [DOI] [PubMed] [Google Scholar]
- FALCONE G., NICKERSON W. J. Cell-wall mannan-protein of baker's yeast. Science. 1956 Aug 10;124(3215):272–273. doi: 10.1126/science.124.3215.272-a. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Hommes F. A. Effect of glucose on the level of glycolytic enzymes in yeast. Arch Biochem Biophys. 1966 Apr;114(1):231–233. doi: 10.1016/0003-9861(66)90325-0. [DOI] [PubMed] [Google Scholar]
- KOLB J. J., WEIDNER M. A., TOENNIES G. Microdetermination of lipid phosphorus as a measure of bacterial membrane substance. Anal Biochem. 1963 Jan;5:78–82. doi: 10.1016/0003-2697(63)90061-7. [DOI] [PubMed] [Google Scholar]
- LIEBLOVA J., BERAN K., STREIBLOVA E. FRACTIONATION OF A POPULATION OF SACCHAROMYCES CEREVISIAE YEASTS BY CENTRIFUGATION IN A DEXTRAN GRADIENT. Folia Microbiol (Praha) 1964 Jul;35:205–213. doi: 10.1007/BF02875838. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MCLELLAN W. L., Jr, LAMPEN J. O. The acid phosphatase of yeast. Localization and secretion by protoplasts. Biochim Biophys Acta. 1963 Feb 12;67:324–326. doi: 10.1016/0006-3002(63)91832-8. [DOI] [PubMed] [Google Scholar]
- Matile P., Moor H., Mühlethaler K. Isolation and properties of the plasmalemma in yeast. Arch Mikrobiol. 1967;58(3):201–211. doi: 10.1007/BF00408804. [DOI] [PubMed] [Google Scholar]
- McMurrough I., Rose A. H. Effect of growth rate and substrate limitation on the composition and structure of the cell wall of Saccharomyces cerevisiae. Biochem J. 1967 Oct;105(1):189–203. doi: 10.1042/bj1050189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza C. G., Villanueva J. R. Preparation and composition of the protoplast membrane of Candida utilis. Biochim Biophys Acta. 1967 May 2;135(2):189–195. doi: 10.1016/0005-2736(67)90113-7. [DOI] [PubMed] [Google Scholar]
- NACHLAS M. M., SELIGMAN A. M. Evidence for the specificity of esterase and lipase by the use of three chromogenic substrates. J Biol Chem. 1949 Nov;181(1):343–355. [PubMed] [Google Scholar]
- NORTHCOTE D. H., HORNE R. W. The chemical composition and structure of the yeast cell wall. Biochem J. 1952 May;51(2):232–236. doi: 10.1042/bj0510232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHILLIPS J. W., LAMANNA C., MALLETTE M. F. GRINDING MICROORGANISMS WITH A PERISTALTIC PUMP. Appl Microbiol. 1965 May;13:460–463. doi: 10.1128/am.13.3.460-463.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RACUSEN D., JOHNSTONE D. B. Estimation of protein in cellular material. Nature. 1961 Jul 29;191:492–493. doi: 10.1038/191492a0. [DOI] [PubMed] [Google Scholar]
- ROTHSTEIN A., JENNINGS D. H., DEMIS C., BRUCE M. The relationship of fermentation to cell structure in yeast. Biochem J. 1959 Jan;71(1):99–106. doi: 10.1042/bj0710099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHATZ G. SUBCELLULAR PARTICLES CARRYING MITOCHONDRIAL ENZYMES IN ANAEROBICALLY-GROWN CELLS OF SACCHAROMYCES CEREVISIAE. Biochim Biophys Acta. 1965 Feb 22;96:342–345. [PubMed] [Google Scholar]
- SUOMALAINEN H., LINKO M., OURA E. Changes in the phosphatase activity of Baker's yeast during the growth phase and location of the phosphatases in the yeast cell. Biochim Biophys Acta. 1960 Jan 29;37:482–490. doi: 10.1016/0006-3002(60)90505-9. [DOI] [PubMed] [Google Scholar]
- SUTTON D. D., LAMPEN J. O. Localization of sucrose and maltose fermenting systems in Saccharomyces cerevisiae. Biochim Biophys Acta. 1962 Jan 29;56:303–312. doi: 10.1016/0006-3002(62)90567-x. [DOI] [PubMed] [Google Scholar]
- TONINO G. J., STEYN-PARVE E. P. Localization of some phosphatases in yeast. Biochim Biophys Acta. 1963 Mar 12;67:453–469. doi: 10.1016/0006-3002(63)91851-1. [DOI] [PubMed] [Google Scholar]
- TORRIANI A. Influence of inorganic phosphate in the formation of phosphatases by Escherichia coli. Biochim Biophys Acta. 1960 Mar 11;38:460–469. doi: 10.1016/0006-3002(60)91281-6. [DOI] [PubMed] [Google Scholar]
- Weimberg R., Orton W. L. Elution of exocellular enzymes from Saccharomyces fragilis and Saccharomyces cerevisiae. J Bacteriol. 1966 Jan;91(1):1–13. doi: 10.1128/jb.91.1.1-13.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]


