Abstract
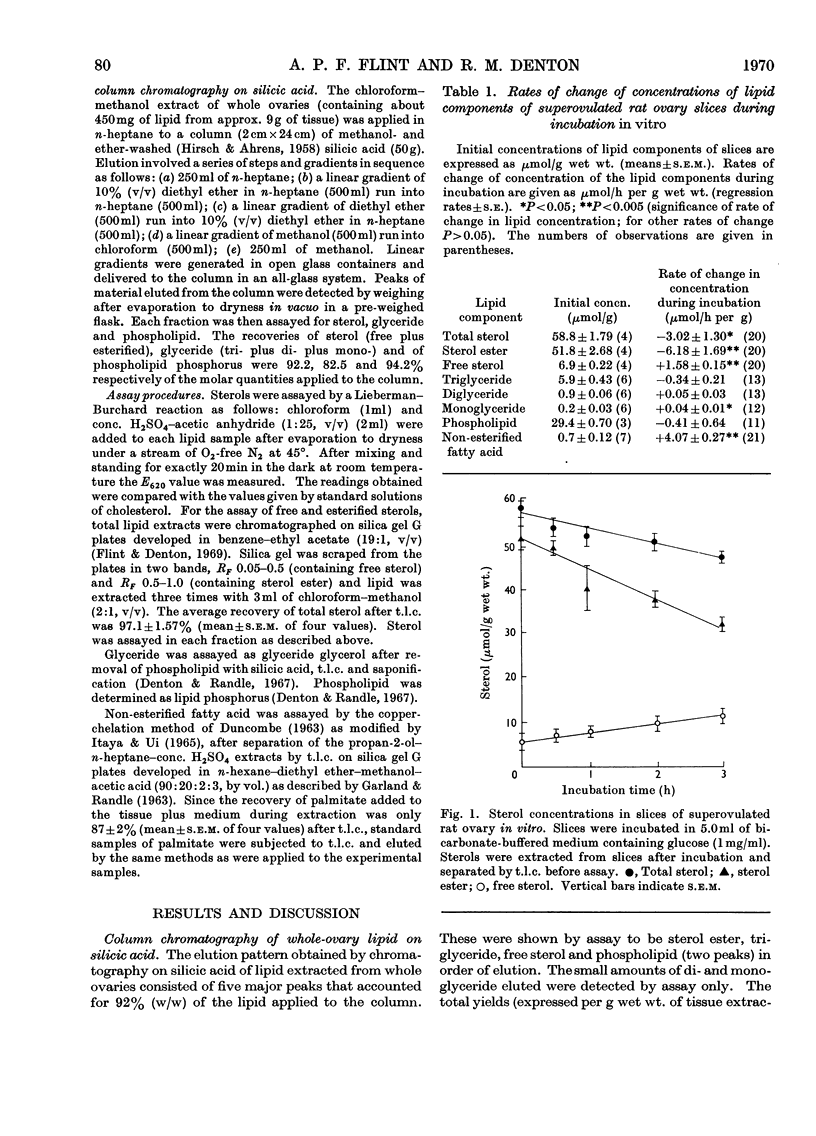
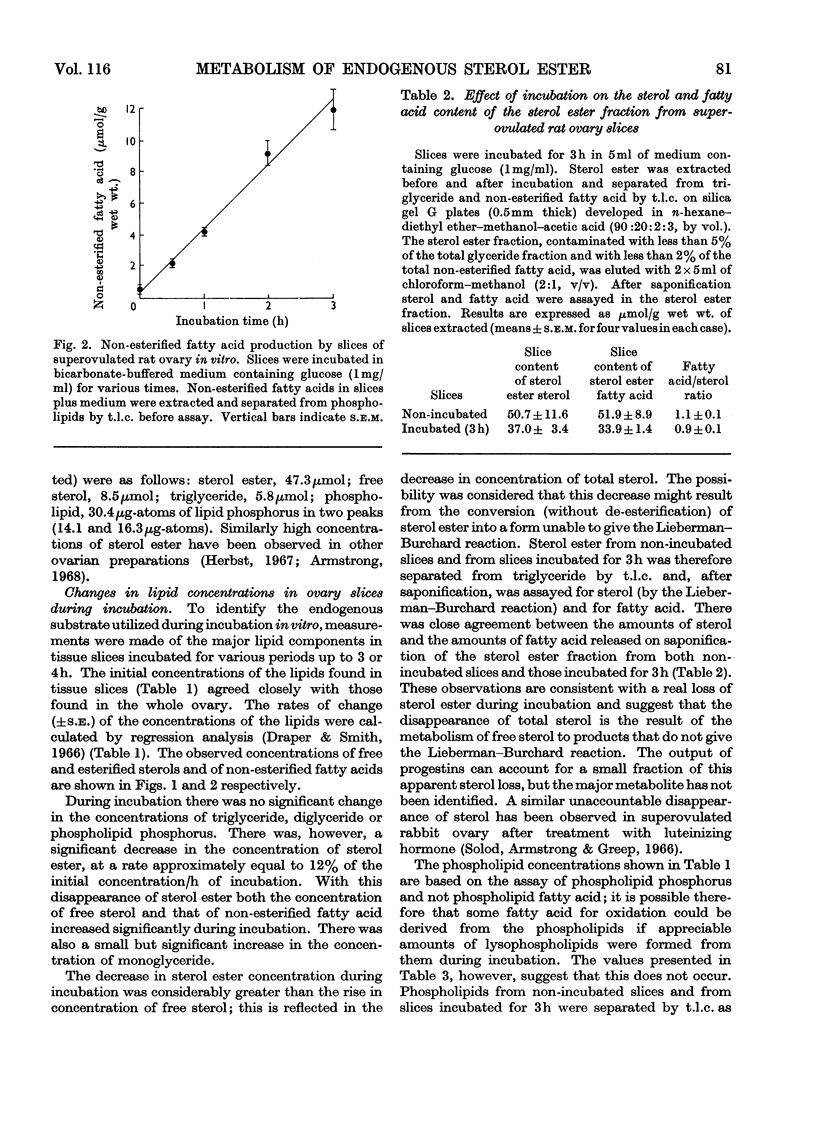
Sterol, glyceride and phospholipid were found to account for more than 90% (w/w) of the lipid extracted from whole superovulated rat ovaries. These lipids, together with non-esterified fatty acids, were assayed in slices of the tissue after incubation for various times. Whereas the concentrations of triglyceride, diglyceride and phospholipid did not change significantly during incubation, that of sterol ester markedly decreased and those of free sterol, monoglyceride and non-esterified fatty acid increased. Evidence is presented that in this tissue (in contrast with other mammalian tissues) the main endogenous substrate for respiration is fatty acid derived from sterol ester.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coutts J. R., Stansfield D. A. Cholesteryl esterase and cholesteryl ester pools in corpus luteum. J Lipid Res. 1968 Sep;9(5):647–651. [PubMed] [Google Scholar]
- DOLE V. P., MEINERTZ H. Microdetermination of long-chain fatty acids in plasma and tissues. J Biol Chem. 1960 Sep;235:2595–2599. [PubMed] [Google Scholar]
- Denton R. M., Randle P. J. Concentrations of glycerides and phospholipids in rat heart and gastrocnemius muscles. Effects of alloxan-diabetes and perfusion. Biochem J. 1967 Aug;104(2):416–422. doi: 10.1042/bj1040416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint A. P., Denton R. M. Glucose metabolism in the superovulated rat ovary in vitro. Effects of luteinizing hormone and the role of glucose metabolism in steroidogenesis. Biochem J. 1969 Apr;112(2):243–254. doi: 10.1042/bj1120243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARLAND P. B., RANDLE P. J. EFFECTS OF ALLOXAN DIABETES AND ADRENALINE ON CONCENTRATIONS OF FREE FATTY ACIDS IN RAT HEART AND DIAPHRAGM MUSCLES. Nature. 1963 Jul 27;199:381–382. doi: 10.1038/199381a0. [DOI] [PubMed] [Google Scholar]
- HIRSCH J., AHRENS E. H., Jr The separation of complex lipide mixtures by the use of silicic acid chromatography. J Biol Chem. 1958 Aug;233(2):311–20. [PubMed] [Google Scholar]
- Herbst A. L. Response of rat ovarian cholesterol to gonadotropins and anterior pituitary hormones. Endocrinology. 1967 Jul;81(1):54–60. doi: 10.1210/endo-81-1-54. [DOI] [PubMed] [Google Scholar]
- Randle P. J., Newsholme E. A., Garland P. B. Regulation of glucose uptake by muscle. 8. Effects of fatty acids, ketone bodies and pyruvate, and of alloxan-diabetes and starvation, on the uptake and metabolic fate of glucose in rat heart and diaphragm muscles. Biochem J. 1964 Dec;93(3):652–665. doi: 10.1042/bj0930652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solod E. A., Armstrong D. T., Greep R. O. Action of luteinizing hormone on conversion of ovarian cholesterol stores to steroids secreted in vivo and synthesized in vitro by the pseudopregnant rabbit ovary. Steroids. 1966 Jun;7(6):607–620. doi: 10.1016/0039-128x(66)90147-4. [DOI] [PubMed] [Google Scholar]
- Stansfield D. A., Flint A. P. The entry of ascorbic acid into the corpus luteum in vivo and in vitro and the effect of luteinizing hormone. J Endocrinol. 1967 Sep;39(1):27–35. doi: 10.1677/joe.0.0390027. [DOI] [PubMed] [Google Scholar]


