Abstract
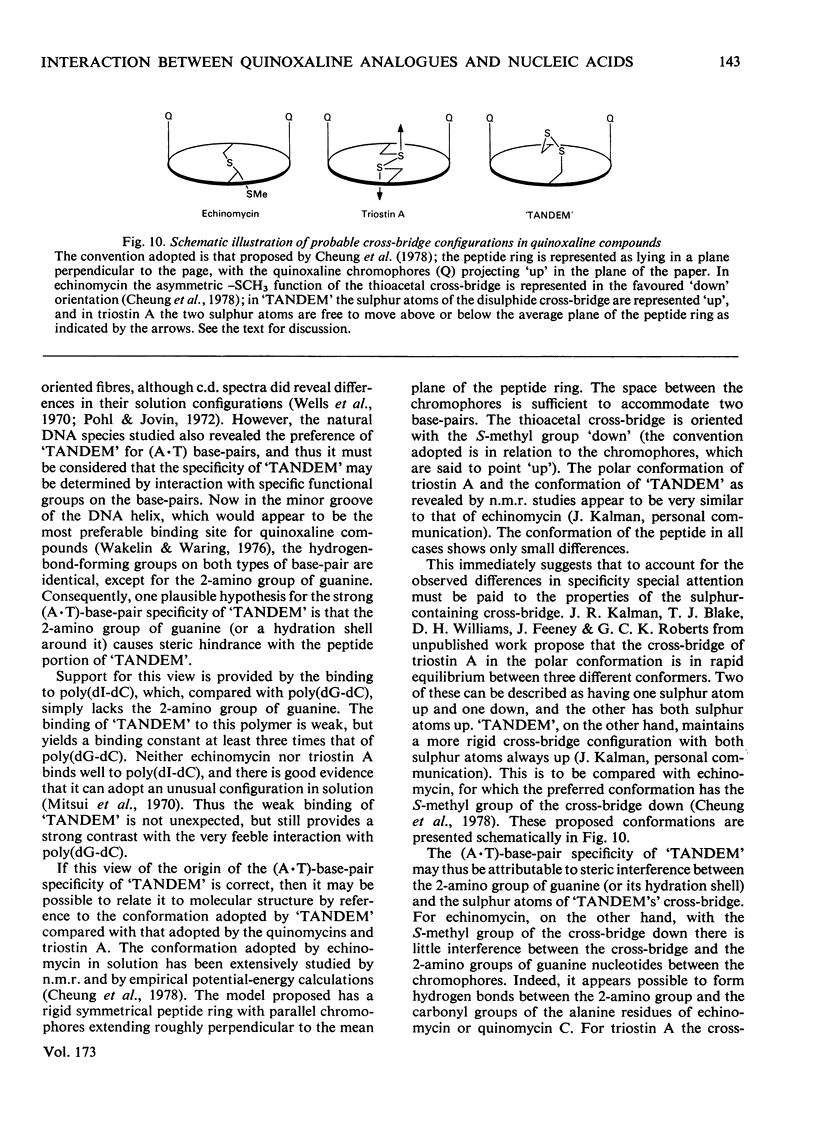
The interaction with DNA of six chemically synthesized derivatives of the quinoxaline antibiotics was investigated. Five of the compounds bound only weakly to DNA or not at all; for these substances spectrophotometric measurements, sedimentation studies with closed circular duplex bacteriophage-PM2 DNA and thermal-denaturation profiles were used to determine limits fot the binding constants. No interaction could be detected with two products of degradation of echinomycin (quinomycin A), one of which, echinomycinic acid dimethyl ester, had the lactone linkages opened, whereas the other retained an intact octapeptide ring but had a broken cross-bridge. The other compounds studied were des-N-tetramethyl-triostin A ('TANDEM') and its derivatives. A derivative of 'TANDEM' IN WHICH benzyloxycarbonyl moieties replace both quinoxaline chromophores had binding constants to nucelic acids in the range 10(2)--10(3)-1, whereas no interaction could be detected for a benzyloxycarbonyl derivative that, in addition, had the cross-bridge broken. The derivative of 'TANDEM' with L-serine in place of D-serine in both positions showed no detectable interaction with Clostridium perfringens DNA, whereas the binding constant to poly(dA-dT) was approx 2 X 10(3)M-1. 'TANDEM' itself bound strongly to DNA, and the bathochromic and hypochromic shifts in its u.v.-absorption spectrum in the presence of DNA were similar to those seen with echinomycin. From the effect on the sedimentation coefficient of closed circular duplex bacteriophage-PM2 DNA the mechanism of binding was shown to involve bifunctional intercalation, typical of the naturally occurring quinoxaline antibiotics. Solvent-partition analysis was used to determine binding constants for the interaction between 'TANDEM' and a variety of natural and synthetic DNA species. The pattern of specificity thus revealed differed markedly from that previously found with the naturally occurring quinoxaline antibiotics. Most striking was the evident large preference for (A + T)-rich DNA species, in complete contrast with echinomycin and triostin A. The highest binding constant was found for poly(dA-dT), the interaction with which appeared highly co-operative in character. The conformations adopted by those quinoxaline compounds that bind strongly to DNA were examined withe aid of molecular models on the basis of results derived from n.m.r. and computer studies. It appears that the observed patterns of base-sequence specificity are determined, at least in part, by the structure and conformation of the sulphur-containing cross-bridge.
Full text
PDF

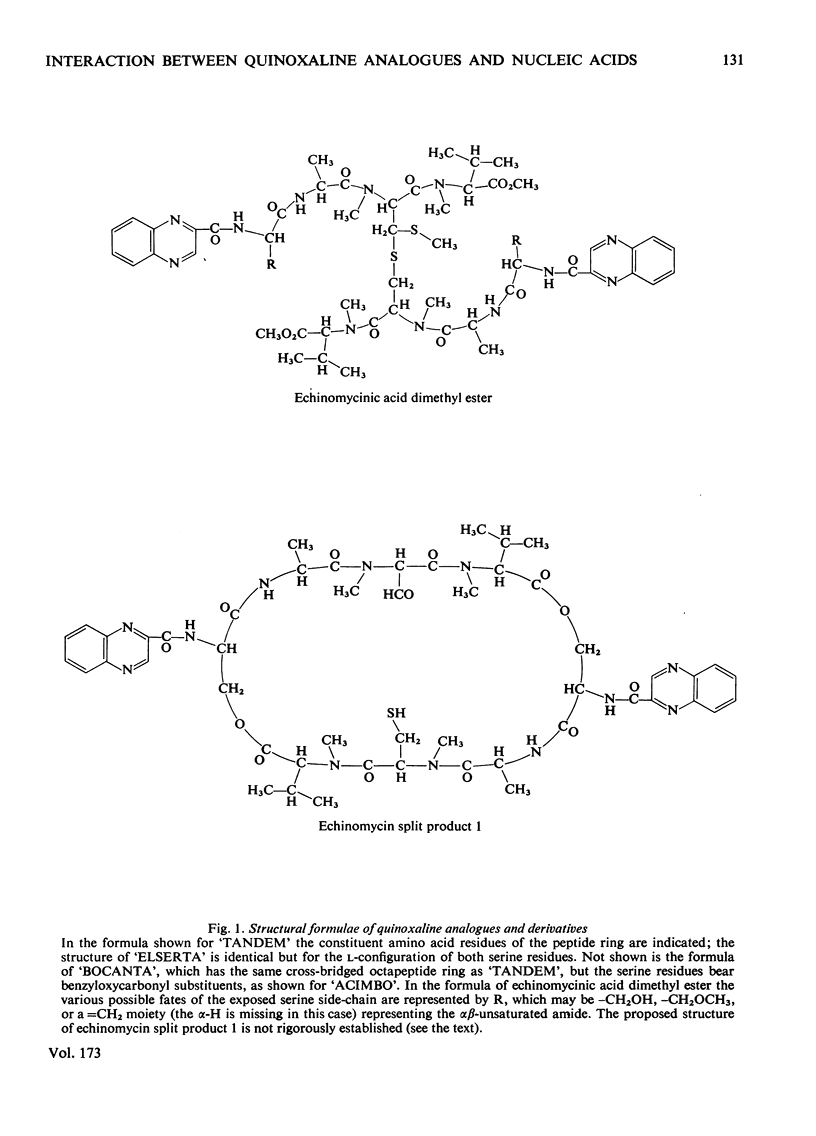




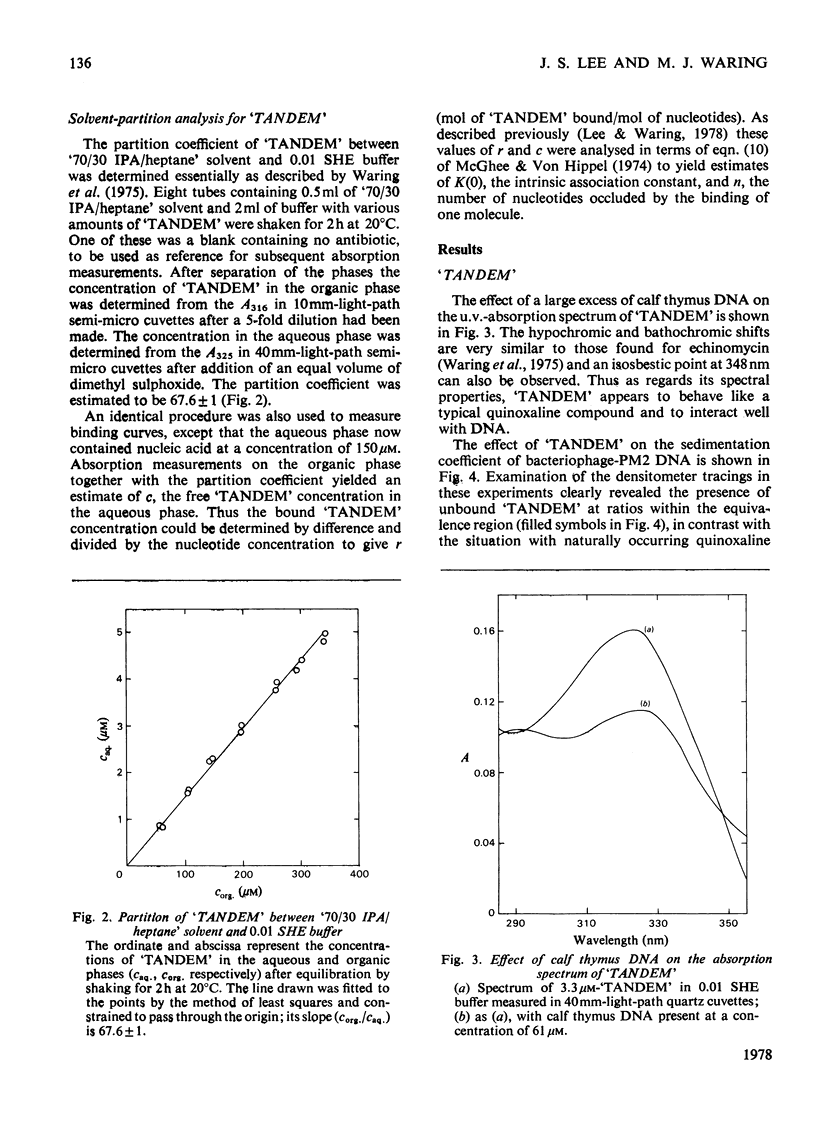
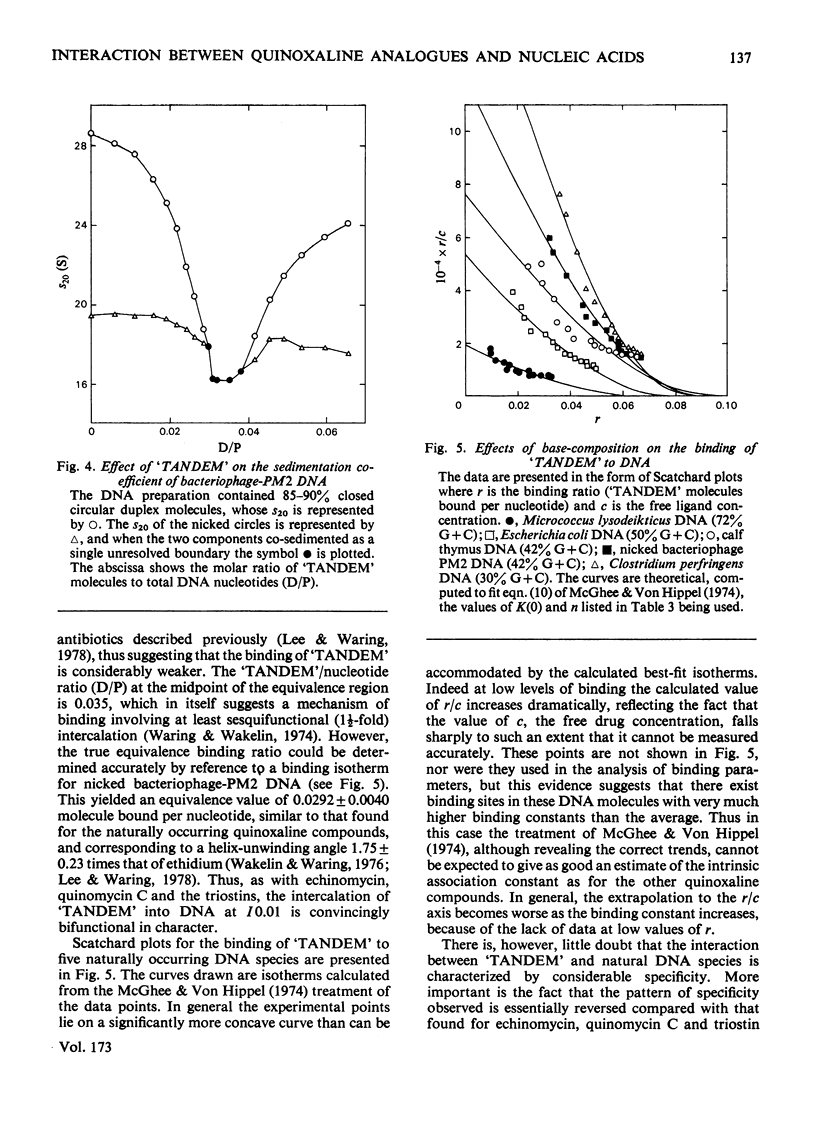
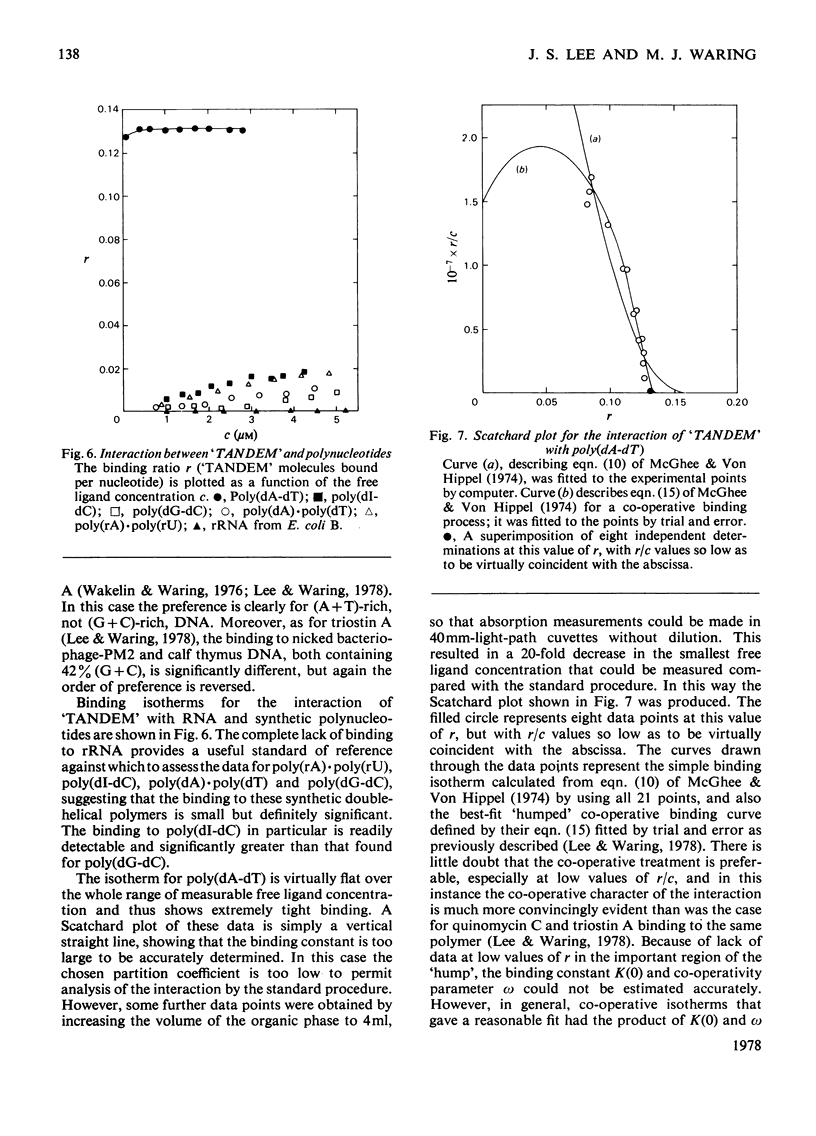





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnott S., Chandrasekaran R., Hukins D. W., Smith P. J., Watts L. Structural details of double-helix observed for DNAs containing alternating purine and pyrimidine sequences. J Mol Biol. 1974 Sep 15;88(2):523–533. doi: 10.1016/0022-2836(74)90499-9. [DOI] [PubMed] [Google Scholar]
- Chen W. Y., Hsu M. L., Olsen R. K. Synthesis of a model depsipeptide lactone related to the quinoxaline antibiotics. J Org Chem. 1975 Oct 17;40(21):3110–3112. doi: 10.1021/jo00909a020. [DOI] [PubMed] [Google Scholar]
- Ciardelli T. L., Olsen R. K. Synthesis of des-N-tetramethyltriostin A, a bicyclic octadepsipeptide related to the quinoxaline antibiotics. J Am Chem Soc. 1977 Apr 13;99(8):2806–2807. doi: 10.1021/ja00450a072. [DOI] [PubMed] [Google Scholar]
- Dell A., Williams D. H., Morris H. R., Smith G. A., Feeney J., Roberts G. C. Structure revision of the antibiotic echinomycin. J Am Chem Soc. 1975 Apr 30;97(9):2497–2502. doi: 10.1021/ja00842a029. [DOI] [PubMed] [Google Scholar]
- Gabbay E. J., Sanford K., Baxter C. S., Kapicak L. Specific interaction of peptides with nucleic acids. Evidence for a "selective bookmark" recognition hypothesis. Biochemistry. 1973 Oct 9;12(21):4021–4029. doi: 10.1021/bi00745a001. [DOI] [PubMed] [Google Scholar]
- Gabbay E. J., Sanford K., Baxter C. S. Specific interaction of peptides with nucleic acids. Biochemistry. 1972 Aug 29;11(18):3429–3435. doi: 10.1021/bi00768a016. [DOI] [PubMed] [Google Scholar]
- Kapicak L., Gabbay E. J. Effect of aromatic cations on the tertiary structure of deoxyribonucleic acid. J Am Chem Soc. 1975 Jan 22;97(2):403–408. doi: 10.1021/ja00835a031. [DOI] [PubMed] [Google Scholar]
- Lee J. S., Waring M. J. Bifunctional intercalation and sequence specificity in the binding of quinomycin and triostin antibiotics to deoxyribonucleic acid. Biochem J. 1978 Jul 1;173(1):115–128. doi: 10.1042/bj1730115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhee J. D. Theoretical calculations of the helix-coil transition of DNA in the presence of large, cooperatively binding ligands. Biopolymers. 1976 Jul;15(7):1345–1375. doi: 10.1002/bip.1976.360150710. [DOI] [PubMed] [Google Scholar]
- McGhee J. D., von Hippel P. H. Theoretical aspects of DNA-protein interactions: co-operative and non-co-operative binding of large ligands to a one-dimensional homogeneous lattice. J Mol Biol. 1974 Jun 25;86(2):469–489. doi: 10.1016/0022-2836(74)90031-x. [DOI] [PubMed] [Google Scholar]
- Mitsui Y., Langridge R., Shortle B. E., Cantor C. R., Grant R. C., Kodama M., Wells R. D. Physical and enzymatic studies on poly d(I-C)-poly d(I-C), an unusual double-helical DNA. Nature. 1970 Dec 19;228(5277):1166–1169. doi: 10.1038/2281166a0. [DOI] [PubMed] [Google Scholar]
- Müller W., Bünemann H., Dattagupta N. Interactions of heteroaromatic compounds with nucleic acids. 2. Influence of substituents on the base and sequence specificity of intercalating ligands. Eur J Biochem. 1975 May;54(1):279–291. doi: 10.1111/j.1432-1033.1975.tb04138.x. [DOI] [PubMed] [Google Scholar]
- Müller W., Crothers D. M. Interactions of heteroaromatic compounds with nucleic acids. 1. The influence of heteroatoms and polarizability on the base specificity of intercalating ligands. Eur J Biochem. 1975 May;54(1):267–277. doi: 10.1111/j.1432-1033.1975.tb04137.x. [DOI] [PubMed] [Google Scholar]
- Müller W., Gautier F. Interactions of heteroaromatic compounds with nucleic acids. A - T-specific non-intercalating DNA ligands. Eur J Biochem. 1975 Jun;54(2):385–394. doi: 10.1111/j.1432-1033.1975.tb04149.x. [DOI] [PubMed] [Google Scholar]
- Pohl F. M., Jovin T. M., Baehr W., Holbrook J. J. Ethidium bromide as a cooperative effector of a DNA structure. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3805–3809. doi: 10.1073/pnas.69.12.3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl F. M., Jovin T. M. Salt-induced co-operative conformational change of a synthetic DNA: equilibrium and kinetic studies with poly (dG-dC). J Mol Biol. 1972 Jun 28;67(3):375–396. doi: 10.1016/0022-2836(72)90457-3. [DOI] [PubMed] [Google Scholar]
- Wakelin S. P., Waring M. J. The binding of echinomycin to deoxyribonucleic acid. Biochem J. 1976 Sep 1;157(3):721–740. doi: 10.1042/bj1570721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring M. J., Wakelin L. P. Echinomycin: a bifunctional intercalating antibiotic. Nature. 1974 Dec 20;252(5485):653–657. doi: 10.1038/252653a0. [DOI] [PubMed] [Google Scholar]
- Waring M. J., Wakelin L. P., Lee J. S. A solvent-partition method for measuring the binding of drugs to DNA. Application to the quinoxaline antibiotics echinomycin and triostin A. Biochim Biophys Acta. 1975 Oct 1;407(2):200–212. doi: 10.1016/0005-2787(75)90285-3. [DOI] [PubMed] [Google Scholar]
- Waring M. Variation of the supercoils in closed circular DNA by binding of antibiotics and drugs: evidence for molecular models involving intercalation. J Mol Biol. 1970 Dec 14;54(2):247–279. doi: 10.1016/0022-2836(70)90429-8. [DOI] [PubMed] [Google Scholar]
- Wells R. D., Larson J. E., Grant R. C., Shortle B. E., Cantor C. R. Physicochemical studies on polydeoxyribonucleotides containing defined repeating nucleotide sequences. J Mol Biol. 1970 Dec 28;54(3):465–497. doi: 10.1016/0022-2836(70)90121-x. [DOI] [PubMed] [Google Scholar]


