Abstract
1. Fragments (2-20 mg wet wt.) of closed needle-biopsy specimens from human liver were disrupted in iso-osmotic sucrose and subjected to low-speed centrifugation. The supernatant was layered on a linear sucrose-density gradient in the Beaufay small-volume automatic zonal rotor. The following organelles, with equilibrium densities (g/ml) and principal marker enzyme shown in parentheses, were resolved: plasma membrane (1.12-1.14; 5'-nucleotidase); lysosomes (1.15-1.20; N-acetyl-beta-glucosaminidase); mitochondria (1.20; malate dehydrogenase); endoplasmic reticulum (1.17-1.21; neutral alpha-glucosidase); peroxisomes (1.22-1.24; catalase). 2. The distribution of particulate alkaline phosphatase and, to a lesser degree, leucine 2-naphthylamidase followed that of 5'-nucleotidase. gamma-Glutamyltransferase was associated with membranes of significantly higher equilibrium density than was 5'-nucleotidase. 3. The distribution of 12 acid hydrolases was determined in the density-gradient fractions. beta-Glucosidase had a predominantly cytosolic localization, but the other enzymes showed a broad distribution of activity throughout the gradient. Evidence was presented for two populations of lysosomes with equilibrium densities of 1.15 and 1.20 g/ml, but containing differing amounts of each enzyme. Further evidence of lysosomal heterogeneity was demonstrated by studying the distribution of isoenzymes of hexosaminidase and of acid phosphatase. 4. The resolving power of the centrifugation procedure can be further enhanced with membrane perturbants. Digitonin (0.12 mM) selectively disrupted lysosomes, markedly increased the equilibrium density of plasma-membrane components and lowered the density of the endoplasmic reticulum, but did not affect the mitochondria or peroxisomes. Pyrophosphate (15 mM) selectively lowered the equilibrium density of the endoplasmic reticulum.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baudhuin P., Beaufay H., Rahman-Li Y., Sellinger O. Z., Wattiaux R., Jacques P., De Duve C. Tissue fractionation studies. 17. Intracellular distribution of monoamine oxidase, aspartate aminotransferase, alanine aminotransferase, D-amino acid oxidase and catalase in rat-liver tissue. Biochem J. 1964 Jul;92(1):179–184. doi: 10.1042/bj0920179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield F. J., Wells G., Welman E., Peters T. J. Analytical subcellular fractionation of guinea-pig myocardium. Clin Sci Mol Med. 1977 Jul;53(1):63–74. doi: 10.1042/cs0530063. [DOI] [PubMed] [Google Scholar]
- Burton R., Lloyd J. B. Latency of some glycosidases of rat liver lysosomes. Biochem J. 1976 Dec 15;160(3):631–638. doi: 10.1042/bj1600631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE DUVE C., WATTIAUX R., BAUDHUIN P. Distribution of enzymes between subcellular fractions in animal tissues. Adv Enzymol Relat Subj Biochem. 1962;24:291–358. doi: 10.1002/9780470124888.ch6. [DOI] [PubMed] [Google Scholar]
- De Duve C., Baudhuin P. Peroxisomes (microbodies and related particles). Physiol Rev. 1966 Apr;46(2):323–357. doi: 10.1152/physrev.1966.46.2.323. [DOI] [PubMed] [Google Scholar]
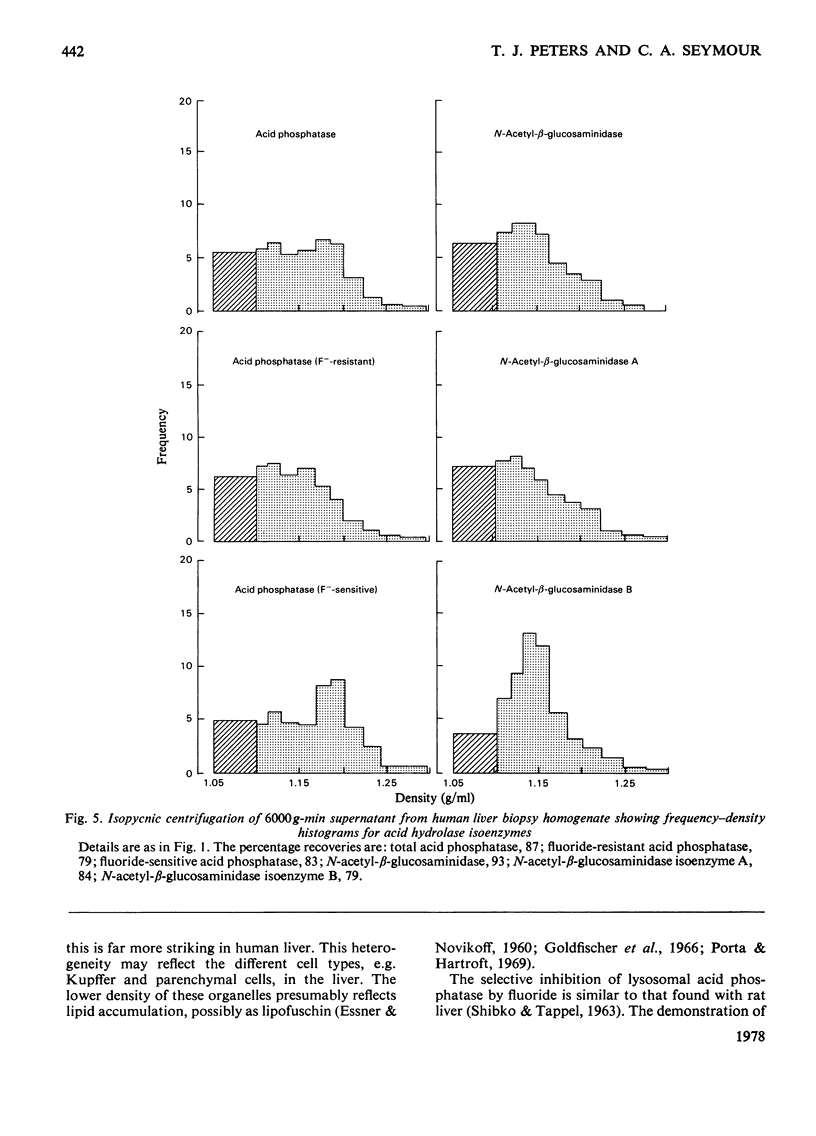
- ESSNER E., NOVIKOFF A. B. Human hepatocellular pigments and lysosomes. J Ultrastruct Res. 1960 Jun;3:374–391. doi: 10.1016/s0022-5320(60)90016-2. [DOI] [PubMed] [Google Scholar]
- Ellis R. B., Ikonne J. U., Masson P. K. DEAE-cellulose microcolumn chromatography coupled with automated assay: application to the resolution of N-acetyl-beta-D-hexosaminidase components. Anal Biochem. 1975 Jan;63(1):5–11. doi: 10.1016/0003-2697(75)90183-9. [DOI] [PubMed] [Google Scholar]
- Fishman W. H., Ide H., Rufo R. Dual localization of acid hydrolases in endoplasmic reticulum and in lysosomes. I. Beta-glucuronidase staining reactions and cytochemical studies on kidney in androgen-stimulated mice. Histochemie. 1969;20(4):287–299. doi: 10.1007/BF00263747. [DOI] [PubMed] [Google Scholar]
- Goldfischer S., Villaverde H., Forschirm R. The demonstration of acid hydrolase, thermostable reduced diphosphopyridine nucleotide tetrazolium reductase and peroxidase activities in human lipofuscin pigment granules. J Histochem Cytochem. 1966 Sep;14(9):641–652. doi: 10.1177/14.9.641. [DOI] [PubMed] [Google Scholar]
- Guilbault G. G., Brignac P., Jr, Zimmer M. Homovanillic acid as a fluorometric substrate for oxidative enzymes. Analytical applications of the peroxidase, glucose oxidase, and xanthine oxidase systems. Anal Chem. 1968 Jan;40(1):190–196. doi: 10.1021/ac60257a002. [DOI] [PubMed] [Google Scholar]
- LEJEUNE N., THINES-SEMPOUX D., HERS H. G. Tissue fractionation studies. 16. Intracellular distribution and properties of alpha-glucosidases in rat liver. Biochem J. 1963 Jan;86:16–21. doi: 10.1042/bj0860016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leighton F., Poole B., Beaufay H., Baudhuin P., Coffey J. W., Fowler S., De Duve C. The large-scale separation of peroxisomes, mitochondria, and lysosomes from the livers of rats injected with triton WR-1339. Improved isolation procedures, automated analysis, biochemical and morphological properties of fractions. J Cell Biol. 1968 May;37(2):482–513. doi: 10.1083/jcb.37.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadevan S., Tappel A. L. Arylamidases of rat liver and kidney. J Biol Chem. 1967 May 25;242(10):2369–2374. [PubMed] [Google Scholar]
- Marsh C. A., Gourlay G. C. Evidence for a non-lysosomal alpha-mannosidase in rat liver homogenates. Biochim Biophys Acta. 1971 Apr 14;235(1):142–148. doi: 10.1016/0005-2744(71)90041-6. [DOI] [PubMed] [Google Scholar]
- Mitropoulos K. A., Venkatesan S., Balasubramaniam S., Peters T. J. The submicrosomal localization of 3-hydroxy-3-methylglutaryl-coenzyme-A reductase, cholesterol 7alpha-hydroxylase and cholesterol in rat liver. Eur J Biochem. 1978 Jan 16;82(2):419–429. doi: 10.1111/j.1432-1033.1978.tb12036.x. [DOI] [PubMed] [Google Scholar]
- Peters T. J. Analytical subcellular fractionation of jejunal biopsy specimens: methodology and characterization of the organelles in normal tissue. Clin Sci Mol Med. 1976 Dec;51(6):557–574. doi: 10.1042/cs0510557. [DOI] [PubMed] [Google Scholar]
- Peters T. J. Application of analytical subcellular fractionation techniques and tissue enzymic analysis to the study of human pathology. Clin Sci Mol Med. 1977 Dec;53(6):505–511. doi: 10.1042/cs0530505. [DOI] [PubMed] [Google Scholar]
- Peters T. J., Batt R. M., Heath J. R., Tilleray J. The micro-assay of intestinal disaccharidases. Biochem Med. 1976 Apr;15(2):145–148. doi: 10.1016/0006-2944(76)90041-7. [DOI] [PubMed] [Google Scholar]
- Peters T. J., De Duve C. Lysosomes of the arterial wall. II. Subcellular fractionation of aortic cells from rabbits with experimantal atheroma. Exp Mol Pathol. 1974 Apr;20(2):228–256. doi: 10.1016/0014-4800(74)90057-4. [DOI] [PubMed] [Google Scholar]
- Peters T. J., Heath J. R., Wansbrough-Jones M. H., Foe W. F. Enzyme activities and properties of lysosomes and brush borders in jejunal biopsies from control subjects and patients with coeliac disease. Clin Sci Mol Med. 1975 Apr;48(4):259–267. doi: 10.1042/cs0480259. [DOI] [PubMed] [Google Scholar]
- Peters T. J., Müller M., De Duve C. Lysosomes of the arterial wall. I. Isolation and subcellular fractionation of cells from normal rabbit aorta. J Exp Med. 1972 Nov 1;136(5):1117–1139. doi: 10.1084/jem.136.5.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters T. J., Shio H. Analytical subcellular fractionation studies on rat liver and on isolated jejunal enterocytes with special reference to the separation of lysosomes, peroxisomes and mitochondria. Clin Sci Mol Med. 1976 May;50(5):355–366. doi: 10.1042/cs0500355. [DOI] [PubMed] [Google Scholar]
- Price R. G., Dance N. The cellular distribution of some rat-kidney glycosidases. Biochem J. 1967 Nov;105(2):877–883. doi: 10.1042/bj1050877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D., Stirling J. L. N-Acetyl-beta-glucosaminidases in human spleen. Biochem J. 1968 Apr;107(3):321–327. doi: 10.1042/bj1070321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J. S., Losty T., Wierbicki E. Assay of proteolytic enzyme activity using a 14C-labeled hemoglobin. Anal Biochem. 1971 Jul;42(1):214–221. doi: 10.1016/0003-2697(71)90029-7. [DOI] [PubMed] [Google Scholar]
- SHIBKO S., TAPPEL A. L. Acid phosphatase of the lysosomal and soluble fraction of rat liver. Biochim Biophys Acta. 1963 May 7;73:76–86. doi: 10.1016/0006-3002(63)90361-5. [DOI] [PubMed] [Google Scholar]
- Schnaitman C., Erwin V. G., Greenawalt J. W. The submitochondrial localization of monoamine oxidase. An enzymatic marker for the outer membrane of rat liver mitochondria. J Cell Biol. 1967 Mar;32(3):719–735. doi: 10.1083/jcb.32.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal A. W., Gregoriadis G., Lavender J. P., Tarin D., Peters T. J. Tissue and hepatic subcellular distribution of liposomes containing bleomycin after intravenous administration to patients with neoplasms. Clin Sci Mol Med. 1976 Oct;51(4):421–425. doi: 10.1042/cs0510421. [DOI] [PubMed] [Google Scholar]
- Seymour C. A., Neale G., Peters T. J. Lysosomal changes in liver tissue from patients with the Dubin-Johnson-Sprinz syndrome. Clin Sci Mol Med. 1977 Mar;52(3):241–248. doi: 10.1042/cs0520241. [DOI] [PubMed] [Google Scholar]
- Seymour C. A., Peters T. J. Enzyme activities in human liver biopsies: assay methods and activities of some lysosomal and membrane-bound enzymes in control tissue and serum. Clin Sci Mol Med. 1977 Mar;52(3):229–239. doi: 10.1042/cs0520229. [DOI] [PubMed] [Google Scholar]
- Solyom A., Trams E. G. Enzyme markers in characterization of isolated plasma membranes. Enzyme. 1972;13(5-6):329–372. doi: 10.1159/000459682. [DOI] [PubMed] [Google Scholar]
- Tilleray J., Peters T. J. Analytical subfractionation of microsomal fractions from the livers of control and Gunn-strain rats. Biochem Soc Trans. 1976;4(2):248–250. doi: 10.1042/bst0040248. [DOI] [PubMed] [Google Scholar]
- Wattiaux R. Behaviour of rat-liver mitochondria during centrifugation in a sucrose gradient. Mol Cell Biochem. 1974 Aug 1;4(1):21–29. doi: 10.1007/BF01731100. [DOI] [PubMed] [Google Scholar]
- Wisher M. H., Evans W. H. Functional polarity of the rat hepatocyte surface membrane. Isolation and characterization of plasma-membrane subfractions from the blood-sinusoidal, bile-Canalicular and contiguous surfaces of the hepatocyte. Biochem J. 1975 Feb;146(2):375–388. doi: 10.1042/bj1460375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wootton A. M., Neale G., Moss D. W. Enzyme activities of cells of different types isolated from livers of normal and cholestatic rats. Clin Sci Mol Med. 1977 Jun;52(6):585–590. doi: 10.1042/cs0520585. [DOI] [PubMed] [Google Scholar]


