Figure 1.
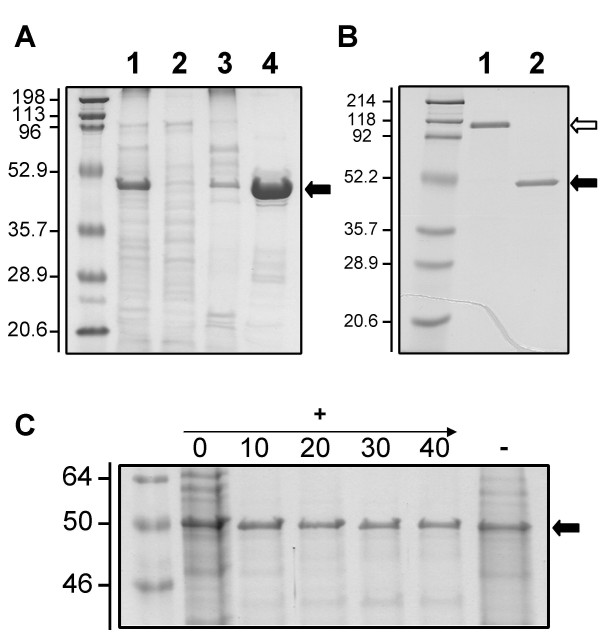
Localization and characterization of OmpJ. A: OmpJ is the most abundant protein in the outer membrane of G. sulfurreducens. Subcellular fractions (cell-free extracts (lane 1), soluble (lane 2), cytoplasmic membrane (lane 3) and outer membrane (lane 4) fractions; 5 μg protein per lane), of G. sulfurreducens grown with fumarate as electron acceptor were analyzed by SDS-PAGE. The most abundant protein in the outer membrane (indicated by an arrow) was designated OmpJ. B: Heat modifiability of OmpJ. OmpJ migrated as a dimer (white arrow) in an SDS-PAGE gel in the absence of heat treatment (lane 1) but migrated as a monomer (solid arrow) after heat treatment at 100°C for 5 minutes (lane 2). Protein, 5 μg per lane. C: Effect of proteinase K treatment on OmpJ integrity. Outer membrane fractions were treated with different concentrations (0 to 40 U ml-1) of proteinase K (+) to analyze the surface exposure of OmpJ and their protein composition was analyzed by denaturing PAGE. A negative control without proteinase K (-) also is shown. First lane in all panels corresponds to molecular weight standards. Numbers at left are molecular masses in kDa.
