Figure 1.
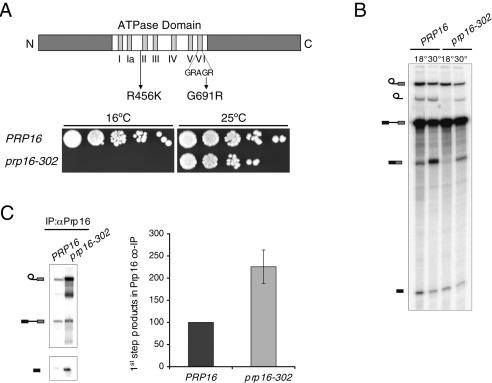
Mutations in the ATPase domain of Prp16p block the second catalytic step and lead to increased association with the spliceosome. (A, top) Schematic diagram of the domain organization of Prp16p. Motifs I–VI in the ATPase domain are represented by vertical bars. The location of the two mutations in the prp16-302 allele is indicated. (Bottom) Serial dilutions of the wild-type PRP16 and mutant prp16-302 strains were spotted on YPD plates and grown for 8 d at the restrictive temperature of 16°C or for 3 d at the permissive temperature of 25°C. (B) In vitro splicing of radiolabeled actin pre-mRNA in extracts from the indicated strains was performed for 30 min at 18°C and subsequently continued for an additional 20 min at 18°C or at 30°C. From top to bottom, the position of the lariat intermediate, lariat intron, precursor, mRNA, and 5′-exon are indicated schematically on the left. (C) Coimmunoprecipitation of splicing intermediates with Prp16p from in vitro splicing reactions performed for 40 min at 18°C. The position of the RNA species is indicated schematically on the left. Unlabeled bands represent degradation products. Signals from at least three independent experiments were quantitated normalizing to levels of the input reaction and expressed relative to the wild-type arbitrarily set to 100.