Abstract
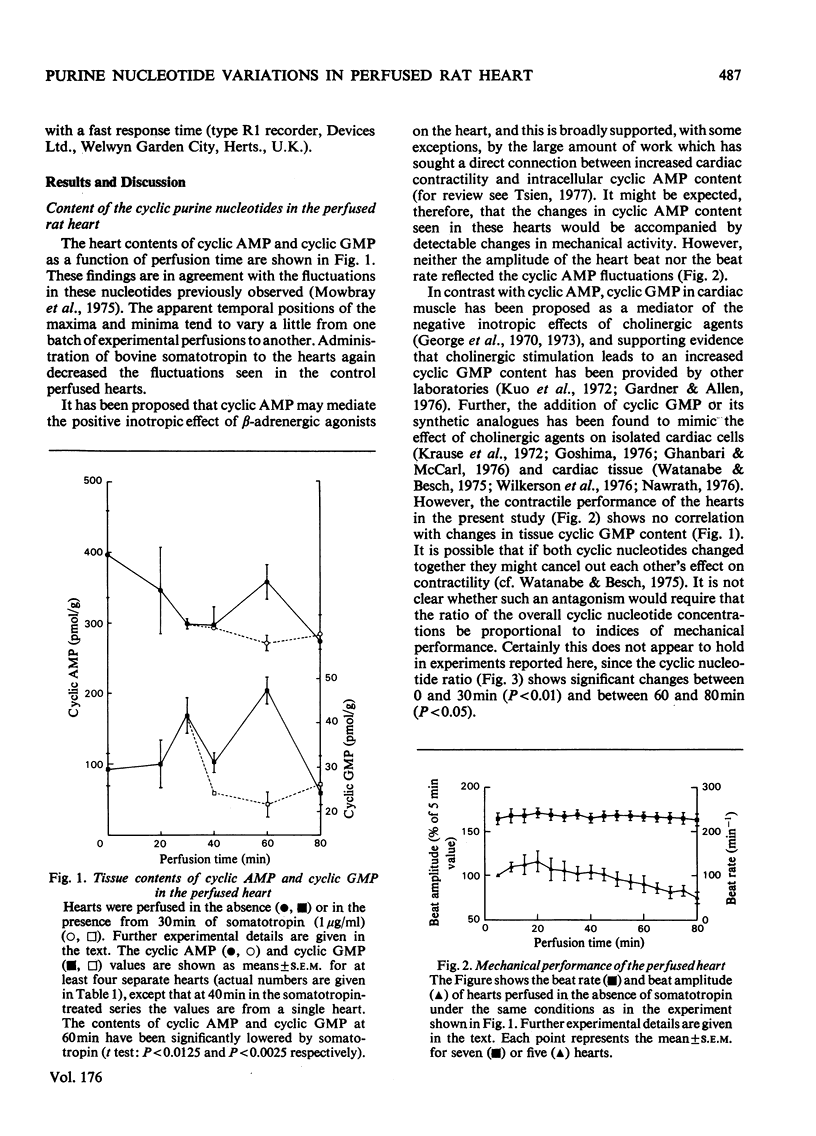
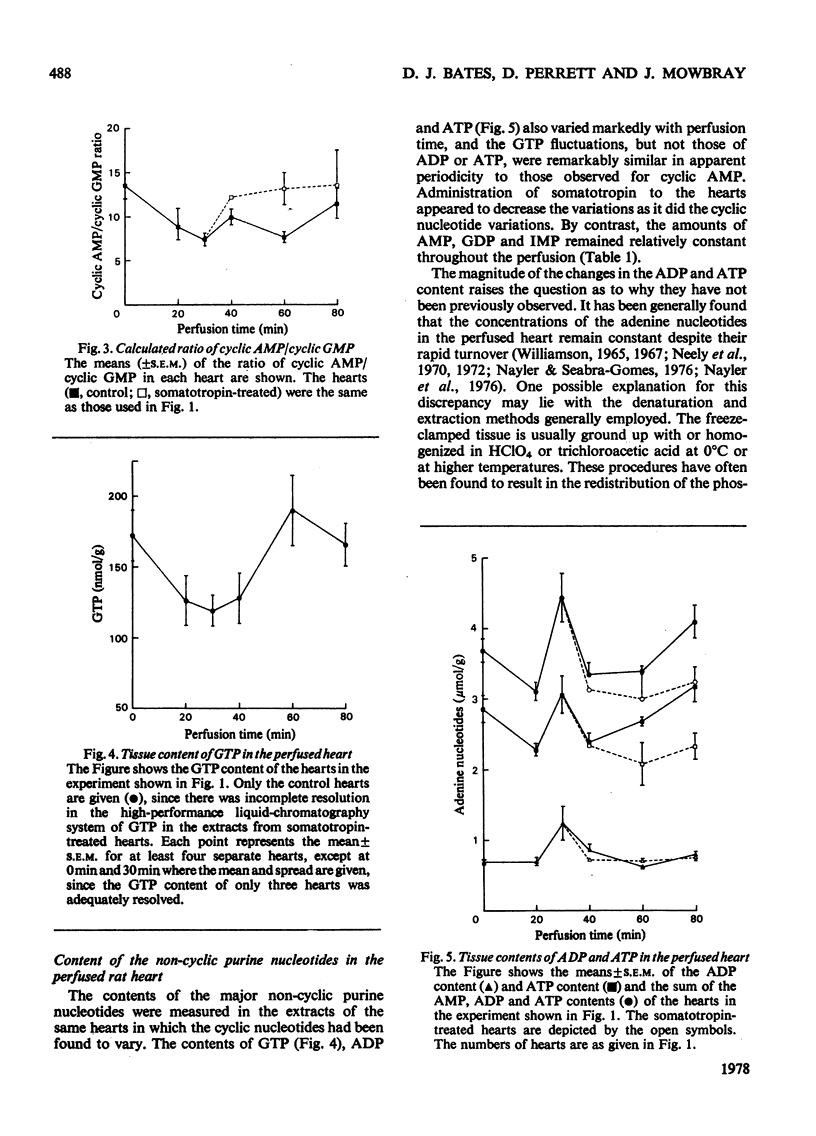
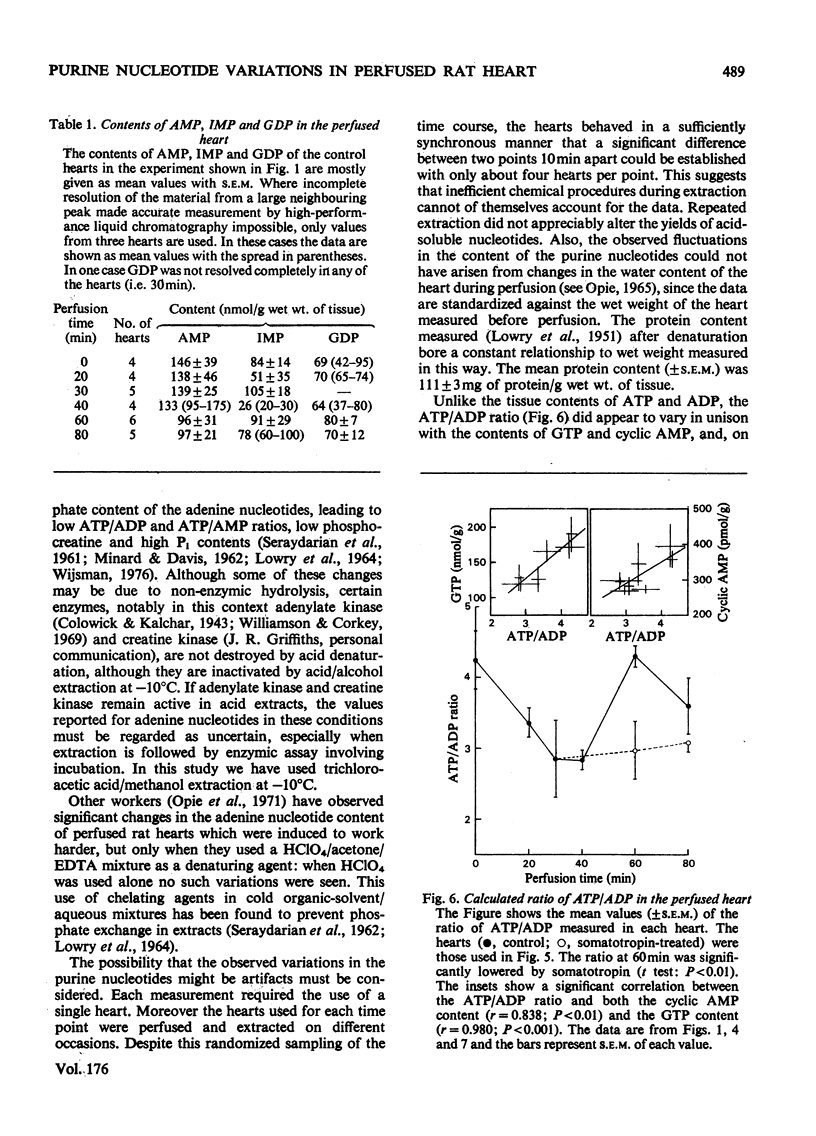
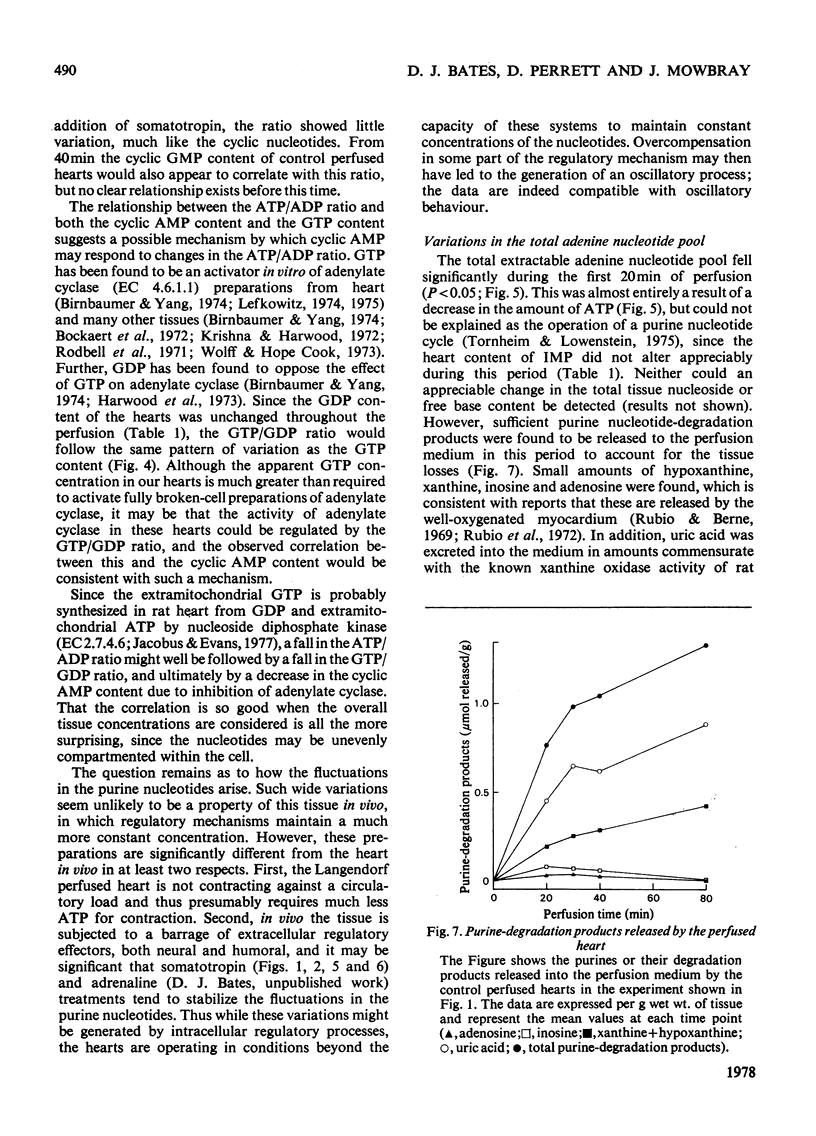
1. The contents of the major purine nucleotides in the isolated non-working perfused rat heart varied systematically during 80min of perfusion. In particular the amounts of ATP, ADP, GTP, cyclic AMP and cyclic GMP in the well-oxygenated myocardium showed changes ranging from 25 to 60% of the mean concentrations. The apparent periodicity was about 30min for some and about 60min for other nucleotides. 2. These data are in contrast with measurements of parameters reflecting heart performance, which remained constant over this period of perfusion. 3. The ATP/ADP ratio, the cyclic AMP content, the GTP content and the GTP/GDP ratio in the tissue bore a constant relationship to one another, and all showed the same temporal variation. 4. Increasing the energy demand on the heart by administration of bovine somatotropin (1μg/ml) tended to damp the variations, and generally lower the content of all the nucleotides. 5. The total extractable adenine nucleotide pool also showed systematic temporal variations of as much as 1.3μmol/g wet wt. of tissue within 10min. 6. These variations could not be accounted for as inter-conversion with adenosine, other purine nucleotides, nucleosides or purine-degradation products either in the tissue or in the perfusion medium. No evidence was found in this preparation of the purine nucleotide oscillations described by Lowenstein and his co-workers [see Tornheim & Lowenstein (1975) J. Biol. Chem. 250, 6304–6314]. 7. Further, the pool size increases cannot be satisfactorily explained by either synthesis de novo or the breakdown of any purine macromolecular species in the cell. Thus it is suggested that an unsuspected substantial storage form of purine nucleotide may exist in heart.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURCH H. B., LOWRY O. H., VONDIPPE P. THE STABILITY OF TRIPHOSPHOPYRIDINE NUCLEOTIDE AND ITS REDUCED FORM IN RAT LIVER. J Biol Chem. 1963 Aug;238:2838–2842. [PubMed] [Google Scholar]
- Birnbaumer L., Yang P. C. Studies on receptor-mediated activation of adenylyl cyclases. III. Regulation by purine nucleotides of the activation of adenylyl cyclases from target organs for prostaglandins, luteinizing hormone, neurohypophyseal hormones and catecholamines. Tissue- and hormone-dependent variations. J Biol Chem. 1974 Dec 25;249(24):7867–7873. [PubMed] [Google Scholar]
- Bockaert J., Roy C., Jard S. Oxytocin-sensitive adenylate cyclase in frog bladder epithelial cells. Role of calcium, nucleotides, and other factors in hormonal stimulation. J Biol Chem. 1972 Nov 10;247(21):7073–7081. [PubMed] [Google Scholar]
- Brown B. L., Albano J. D., Ekins R. P., Sgherzi A. M. A simple and sensitive saturation assay method for the measurement of adenosine 3':5'-cyclic monophosphate. Biochem J. 1971 Feb;121(3):561–562. doi: 10.1042/bj1210561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunschede H., Krooth R. S. Studies on the xanthine oxidase activity of mammalian cells. Biochem Genet. 1973 Apr;8(4):341–350. doi: 10.1007/BF00487339. [DOI] [PubMed] [Google Scholar]
- Burch H. B., Bradley M. E., Lowry O. H. The measurement of triphosphopyridine nucleotide and reduced triphosphopyridine nucleotide and the role of hemoglobin in producing erroneous triphosphopyridine nucleotide values. J Biol Chem. 1967 Oct 10;242(19):4546–4554. [PubMed] [Google Scholar]
- Clegg J. S., Warner A. H., Finamore F. J. Evidence for the function of P-1, P-4-diguanosine 5'-tetraphosphate in the development of Artemia salina. J Biol Chem. 1967 Apr 25;242(8):1938–1943. [PubMed] [Google Scholar]
- FINAMORE F. J., WARNER A. H. The occurrence of P1, P4-diguanosine 5'-tetraphosphate in brine shrimp eggs. J Biol Chem. 1963 Jan;238:344–348. [PubMed] [Google Scholar]
- GOLDTHWAIT D. A. Mechanisms of synthesis of purine nucleotides in heart muscle extracts. J Clin Invest. 1957 Nov;36(11):1572–1578. doi: 10.1172/JCI103555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner R. M., Allen D. O. Effect of acetylcholine on glycogen phosphorylase activity and cyclic nucleotide content in isolated perfused rat hearts. J Cyclic Nucleotide Res. 1976;2(3):171–178. [PubMed] [Google Scholar]
- George W. J., Polson J. B., O'Toole A. G., Goldberg N. D. Elevation of guanosine 3',5'-cyclic phosphate in rat heart after perfusion with acetylcholine. Proc Natl Acad Sci U S A. 1970 Jun;66(2):398–403. doi: 10.1073/pnas.66.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George W. J., Wilkerson R. D., Kadowitz P. J. Influence of acetylcholine on contractile force and cyclic nucleotide levels in the isolated perfused rat heart. J Pharmacol Exp Ther. 1973 Jan;184(1):228–235. [PubMed] [Google Scholar]
- Gerlach E., Zimmer H. G. Alterations of myocardial adenine nucleotide metabolism. Recent Adv Stud Cardiac Struct Metab. 1975;7:121–130. [PubMed] [Google Scholar]
- Ghanbari H., McCarl R. L. Involvement of cyclic nucleotides in the beating response of rat heart cells in culture. J Mol Cell Cardiol. 1976 Jun;8(6):481–488. doi: 10.1016/0022-2828(76)90021-3. [DOI] [PubMed] [Google Scholar]
- Goh S. H., Léjohn H. B. Genetical and biochemical evidence that a novel dinucleoside polyphosphate coordinates salvage and de novo nucleotide biosynthetic pathways in mammalian cells. Biochem Biophys Res Commun. 1977 Jan 10;74(1):256–264. doi: 10.1016/0006-291x(77)91402-4. [DOI] [PubMed] [Google Scholar]
- Goshima K. Antagonistic influences of dibutyryl cyclic AMP and dibutyryl cyclic GMP on the beating rate of cultured mouse myocardial cells. J Mol Cell Cardiol. 1976 Sep;08(9):713–725. doi: 10.1016/0022-2828(76)90013-4. [DOI] [PubMed] [Google Scholar]
- Harwood J. P., Löw H., Rodbell M. Stimulatory and inhibitory effects of guanyl nucleotides on fat cell adenylate cyclase. J Biol Chem. 1973 Sep 10;248(17):6239–6245. [PubMed] [Google Scholar]
- Hess B., Boiteux A., Krüger J. Cooperation of glycolytic enzymes. Adv Enzyme Regul. 1969;7:149–167. doi: 10.1016/0065-2571(69)90016-8. [DOI] [PubMed] [Google Scholar]
- Isselhard W., Hinzen D. H., Geppert E., Mäurer W. Beeinflussung des post-asphyktischen Wiederaufblaues der Adeninnucleotide in Kaninchenherzen in vivo durch Substratangebot. Pflugers Arch. 1970;320(3):195–209. doi: 10.1007/BF00587453. [DOI] [PubMed] [Google Scholar]
- Isselhard W., Mäurer W., Stremmel W., Krebs J., Schmitz H., Neuhof H., Esser A. Stoffwechsel des Kaninchenherzens in situ während Asphyxie und in der post-asphyktischen Erholung. Pflugers Arch. 1970;316(2):164–193. doi: 10.1007/BF00586484. [DOI] [PubMed] [Google Scholar]
- Jacobus W. E., Evans J. J. Nucleoside diphosphokinase of rat heart mitochondria. Dual localization in matrix and intermembrane space. J Biol Chem. 1977 Jun 25;252(12):4232–4241. [PubMed] [Google Scholar]
- Kolassa N., Pfleger K., Rummel W. Specificity of adenosine uptake into the heart and inhibition by dipyridamole. Eur J Pharmacol. 1970 Mar;9(3):265–268. doi: 10.1016/0014-2999(70)90221-9. [DOI] [PubMed] [Google Scholar]
- Krause E. G., Halle W., Wollenberger A. Effect of dibutyryl cyclic GMP on cultured beating rat heart cells. Adv Cyclic Nucleotide Res. 1972;1:301–305. [PubMed] [Google Scholar]
- Krishna G., Harwood J. P. Requirement for guanosine triphosphate in the prostaglandin activation of adenylate cyclase of platelet membranes. J Biol Chem. 1972 Apr 10;247(7):2253–2254. [PubMed] [Google Scholar]
- Kuo J. F., Lee T. P., Reyes P. L., Walton K. G., Donnelly T. E., Jr, Greengard P. Cyclic nucleotide-dependent protein kinases. X. An assay method for the measurement of quanosine 3',5'-monophosphate in various biological materials and a study of agents regulating its levels in heart and brain. J Biol Chem. 1972 Jan 10;247(1):16–22. [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V., HASSELBERGER F. X., SCHULZ D. W. EFFECT OF ISCHEMIA ON KNOWN SUBSTRATES AND COFACTORS OF THE GLYCOLYTIC PATHWAY IN BRAIN. J Biol Chem. 1964 Jan;239:18–30. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lefkowitz R. J. Stimulation of catecholamine-sensitive adenylate cyclase by 5'-guanylyl-imidodiphosphate. J Biol Chem. 1974 Oct 10;249(19):6119–6124. [PubMed] [Google Scholar]
- Liu M. S., Feinberg H. Incorporation of adenosine-8-14/C and inosine-8-14C into rabbit heart adenine nucleotides. Am J Physiol. 1971 May;220(5):1242–1248. doi: 10.1152/ajplegacy.1971.220.5.1242. [DOI] [PubMed] [Google Scholar]
- Maguire M. H., Lukas M. C., Rettie J. F. Adenine nucleotide salvage synthesis in the rat heart; pathways of adnosine salvage. Biochim Biophys Acta. 1972 Mar 14;262(2):108–115. doi: 10.1016/0005-2787(72)90223-7. [DOI] [PubMed] [Google Scholar]
- Mowbray J., Davies J. A., Bates D. J., Jones C. J. Growth hormone, cyclic nucleotides and the rapid control of translation in heart muscle. Biochem J. 1975 Dec;152(3):583–592. doi: 10.1042/bj1520583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowbray J., Last K. S. Evidence against the incorporation into protein of amino acids directly from the membrane transport system in rat heart. Biochim Biophys Acta. 1974 Apr 27;349(1):114–122. doi: 10.1016/0005-2787(74)90014-8. [DOI] [PubMed] [Google Scholar]
- Mowbray J., Ottaway J. H. The effect of insulin and growth hormone on the flux of tracer from labelled lactate in perfused rat heart. Eur J Biochem. 1973 Jul 16;36(2):369–379. doi: 10.1111/j.1432-1033.1973.tb02921.x. [DOI] [PubMed] [Google Scholar]
- Mowbray J., Ottaway J. H. The flux of pyruvate in perfused rat heart. Eur J Biochem. 1973 Jul 16;36(2):362–368. doi: 10.1111/j.1432-1033.1973.tb02920.x. [DOI] [PubMed] [Google Scholar]
- Nawrath H. Cyclic AMP and cyclic GMP may play opposing roles in influencing force of contraction in mammalian myocardium. Nature. 1976 Aug 5;262(5568):509–511. doi: 10.1038/262509b0. [DOI] [PubMed] [Google Scholar]
- Nayler W. G., Grau A., Slade A. A protective effect of verapamil on hypoxic heart muscle. Cardiovasc Res. 1976 Nov;10(6):650–662. doi: 10.1093/cvr/10.6.650. [DOI] [PubMed] [Google Scholar]
- Nayler W. G., Seabra-Gomes R. Effect of methylprednisolone sodium succinate on hypoxic heart muscle. Cardiovasc Res. 1976 May;10(3):349–358. doi: 10.1093/cvr/10.3.349. [DOI] [PubMed] [Google Scholar]
- Neely J. R., Denton R. M., England P. J., Randle P. J. The effects of increased heart work on the tricarboxylate cycle and its interactions with glycolysis in the perfused rat heart. Biochem J. 1972 Jun;128(1):147–159. doi: 10.1042/bj1280147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely J. R., Whitfield C. F., Morgan H. E. Regulation of glycogenolysis in hearts: effects of pressure development, glucose, and FFA. Am J Physiol. 1970 Oct;219(4):1083–1088. doi: 10.1152/ajplegacy.1970.219.4.1083. [DOI] [PubMed] [Google Scholar]
- Oikawa T. G., Smith M. Nucleotides in the encysted embryos of Daphnia magna. Biochemistry. 1966 May;5(5):1517–1521. doi: 10.1021/bi00869a010. [DOI] [PubMed] [Google Scholar]
- Olsson R. A. Changes in content of purine nucleoside in canine myocardium during coronary occlusion. Circ Res. 1970 Mar;26(3):301–306. doi: 10.1161/01.res.26.3.301. [DOI] [PubMed] [Google Scholar]
- Opie L. H. Coronary flow rate and perfusion pressure as determinants of mechanical function and oxidative metabolism of isolated perfused rat heart. J Physiol. 1965 Oct;180(3):529–541. doi: 10.1113/jphysiol.1965.sp007715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opie L. H., Mansford K. R., Owen P. Effects of increased heart work on glycolysis and adenine nucleotides in the perfused heart of normal and diabetic rats. Biochem J. 1971 Sep;124(3):475–490. doi: 10.1042/bj1240475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrett D. Simplified low-pressure high-resolution nucleotide analyser. J Chromatogr. 1976 Sep 15;124(2):187–196. doi: 10.1016/s0021-9673(00)89735-6. [DOI] [PubMed] [Google Scholar]
- Rodbell M., Birnbaumer L., Pohl S. L., Krans H. M. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. V. An obligatory role of guanylnucleotides in glucagon action. J Biol Chem. 1971 Mar 25;246(6):1877–1882. [PubMed] [Google Scholar]
- Rubio R., Berne R. M. Release of adenosine by the normal myocardium in dogs and its relationship to the regulation of coronary resistance. Circ Res. 1969 Oct;25(4):407–415. doi: 10.1161/01.res.25.4.407. [DOI] [PubMed] [Google Scholar]
- Rubio V. R., Wiedmeier T., Berne R. M. Nucleoside phosphorylase: localization and role in the myocardial distribution of purines. Am J Physiol. 1972 Mar;222(3):550–555. doi: 10.1152/ajplegacy.1972.222.3.550. [DOI] [PubMed] [Google Scholar]
- SERAYDARIAN K., MOMMAERTS W. F., WALLNER A., GUILLORY R. J. An estimation of the true inorganic phosphate content of frog sartorius muscle. J Biol Chem. 1961 Jul;236:2071–2075. [PubMed] [Google Scholar]
- SERAYDARIAN K., MOMMAERTS W. F., WALLNER A. The amount and compartmentalization of adenosine diphosphate in muscle. Biochim Biophys Acta. 1962 Dec 17;65:443–460. doi: 10.1016/0006-3002(62)90447-x. [DOI] [PubMed] [Google Scholar]
- Stone P. R., Bredehorst R., Kittler M., Lengyel H., Hilz H. Quantitative determination of poly(adenosine diphosphate ribose) in different hepatic tissues by an isotope dilution procedure. Hoppe Seylers Z Physiol Chem. 1976 Jan;357(1):51–56. doi: 10.1515/bchm2.1976.357.1.51. [DOI] [PubMed] [Google Scholar]
- Stone P. R., Hilz H. Quantitation of hydroxylamine sensitive mono(adenosine diphosphate ribose) residues in different hepatic tissues. FEBS Lett. 1975 Sep 15;57(2):209–212. doi: 10.1016/0014-5793(75)80718-6. [DOI] [PubMed] [Google Scholar]
- Tornheim K., Lowenstein J. M. The purine nucleotide cycle. Control of phosphofructokinase and glycolytic oscillations in muscle extracts. J Biol Chem. 1975 Aug 25;250(16):6304–6314. [PubMed] [Google Scholar]
- Tovey K. C., Oldham K. G., Whelan J. A. A simple direct assay for cyclic AMP in plasma and other biological samples using an improved competitive protein binding technique. Clin Chim Acta. 1974 Nov 8;56(3):221–234. doi: 10.1016/0009-8981(74)90133-8. [DOI] [PubMed] [Google Scholar]
- Tsien R. W. Cyclic AMP and contractile activity in heart. Adv Cyclic Nucleotide Res. 1977;8:363–420. [PubMed] [Google Scholar]
- WILLIAMSON J. R. GLYCOLYTIC CONTROL MECHANISMS. I. INHIBITION OF GLYCOLYSIS BY ACETATE AND PYRUVATE IN THE ISOLATED, PERFUSED RAT HEART. J Biol Chem. 1965 Jun;240:2308–2321. [PubMed] [Google Scholar]
- Warner A. H., Finamore F. J. Isolation, purification, and characterization of P1,P3-diguanosine 5'-triphosphate from brine shrimp eggs. Biochim Biophys Acta. 1965 Dec 9;108(4):525–530. doi: 10.1016/0005-2787(65)90049-3. [DOI] [PubMed] [Google Scholar]
- Warner A. H., McClean D. K. Studies on the biosynthesis and role of diguanosine tetraphosphate during growth and development of Artemia salina. Dev Biol. 1968 Sep;18(3):278–293. doi: 10.1016/0012-1606(68)90036-5. [DOI] [PubMed] [Google Scholar]
- Warner A. H., Thomas D. D., Shridhar V., McCurdy H. D. The diguanosine nucleotides: do they exist in aquatic fungi? Can J Biochem. 1977 Aug;55(8):841–846. doi: 10.1139/o77-124. [DOI] [PubMed] [Google Scholar]
- Watanabe A. M., Besch H. R., Jr Interaction between cyclic adenosine monophosphate and cyclic gunaosine monophosphate in guinea pig ventricular myocardium. Circ Res. 1975 Sep;37(3):309–317. doi: 10.1161/01.res.37.3.309. [DOI] [PubMed] [Google Scholar]
- Wilkerson R. D., Paddock R. J., George W. J. Effects of derivatives of cyclic amp and cyclic gmp on contraction force of cat papillary muscles. Eur J Pharmacol. 1976 Mar;36(1):247–251. doi: 10.1016/0014-2999(76)90280-6. [DOI] [PubMed] [Google Scholar]
- Williamson J. R. Glycolytic control mechanisms. 3. Effects of iodoacetamide and fluoroacetate on glucose metabolism in the perfused rat heart. J Biol Chem. 1967 Oct 10;242(19):4476–4485. [PubMed] [Google Scholar]
- Wolff J., Cook G. H. Activation of thyroid membrane adenylate cyclase by purine nucleotides. J Biol Chem. 1973 Jan 10;248(1):350–355. [PubMed] [Google Scholar]
- Zimmer H. G., Trendelenburg C., Kammermeier H., Gerlach E. De novo synthesis of myocardial adenine nucleotides in the rat. Acceleration during recovery from oxygen deficiency. Circ Res. 1973 May;32(5):635–642. doi: 10.1161/01.res.32.5.635. [DOI] [PubMed] [Google Scholar]


