Abstract
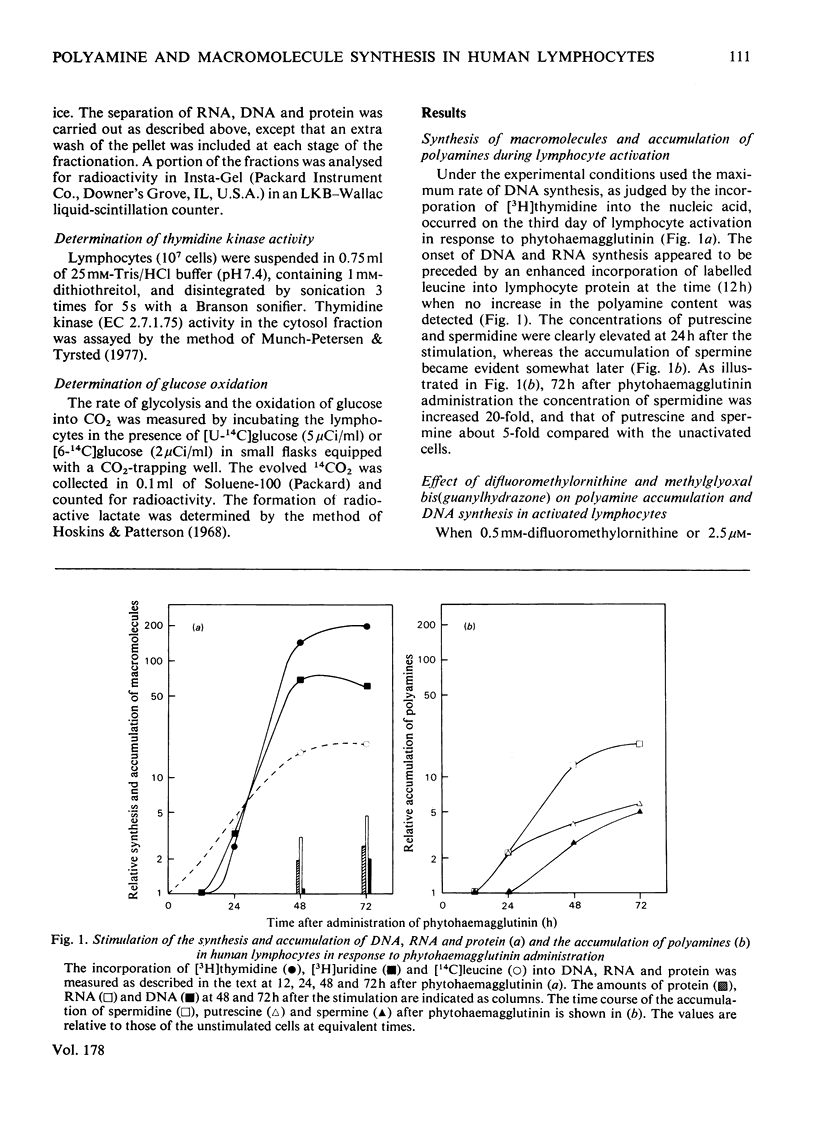
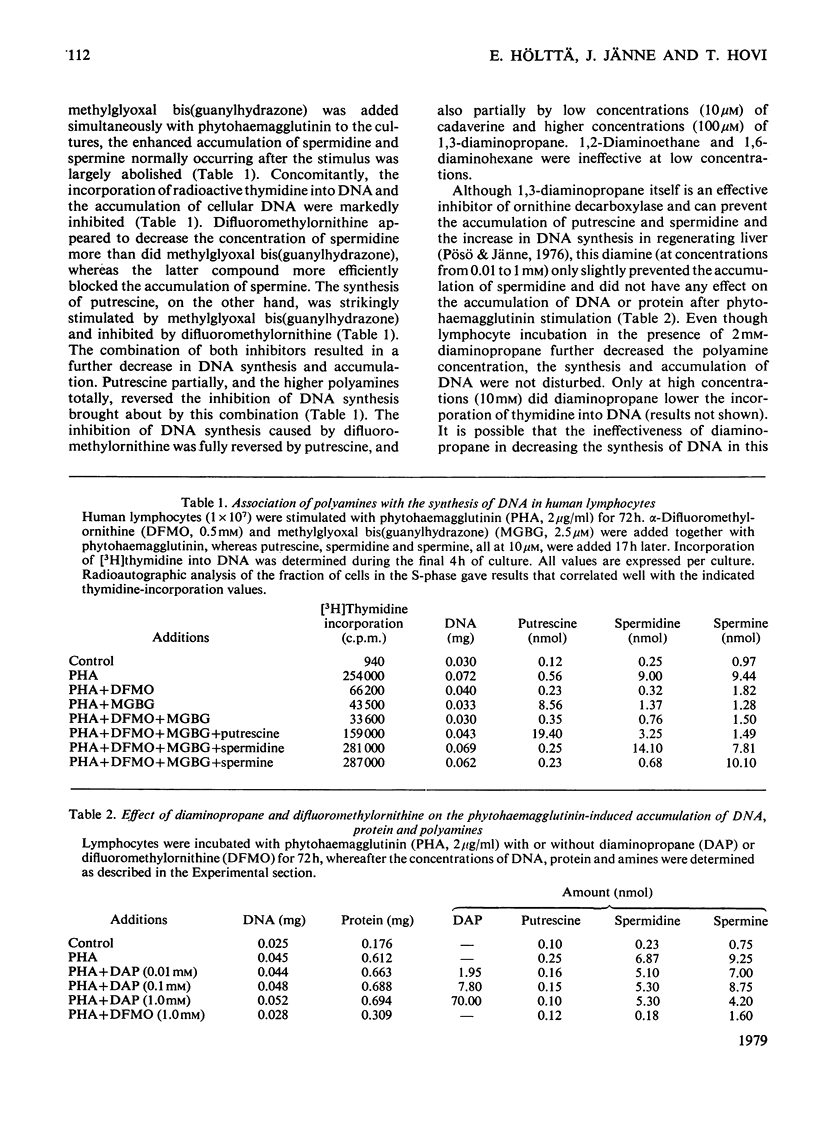
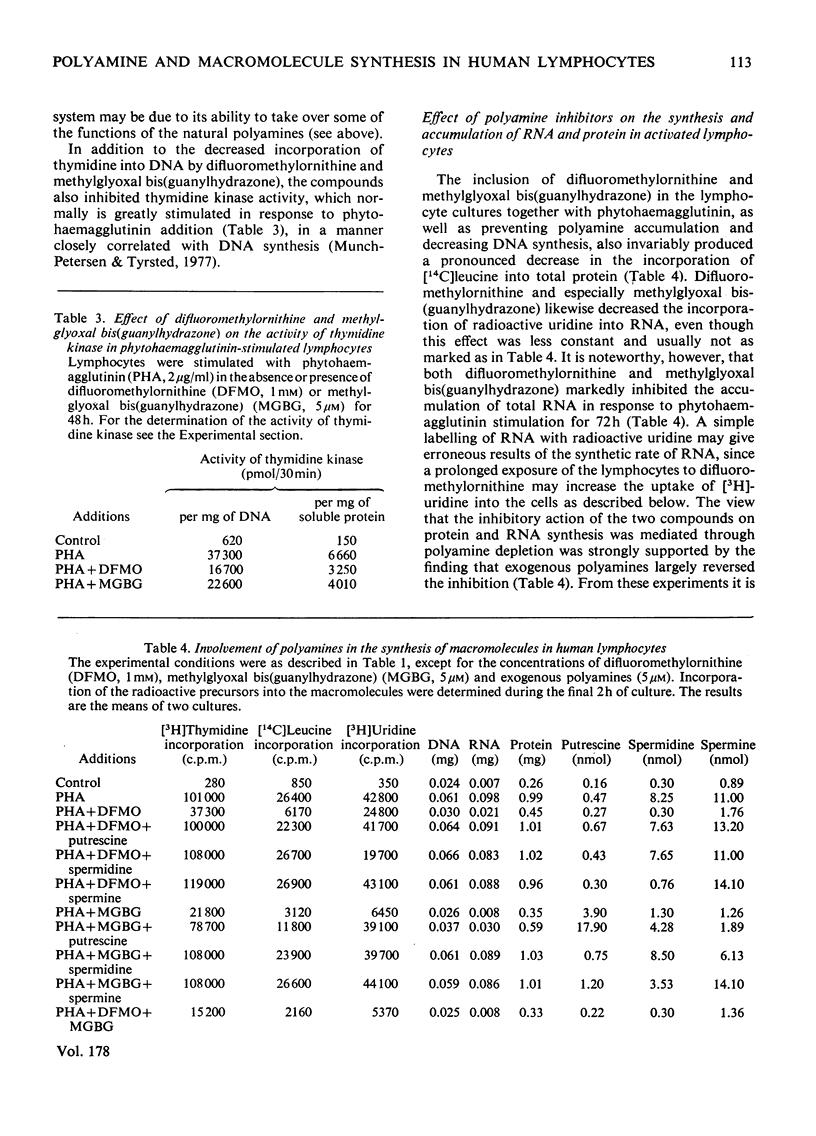
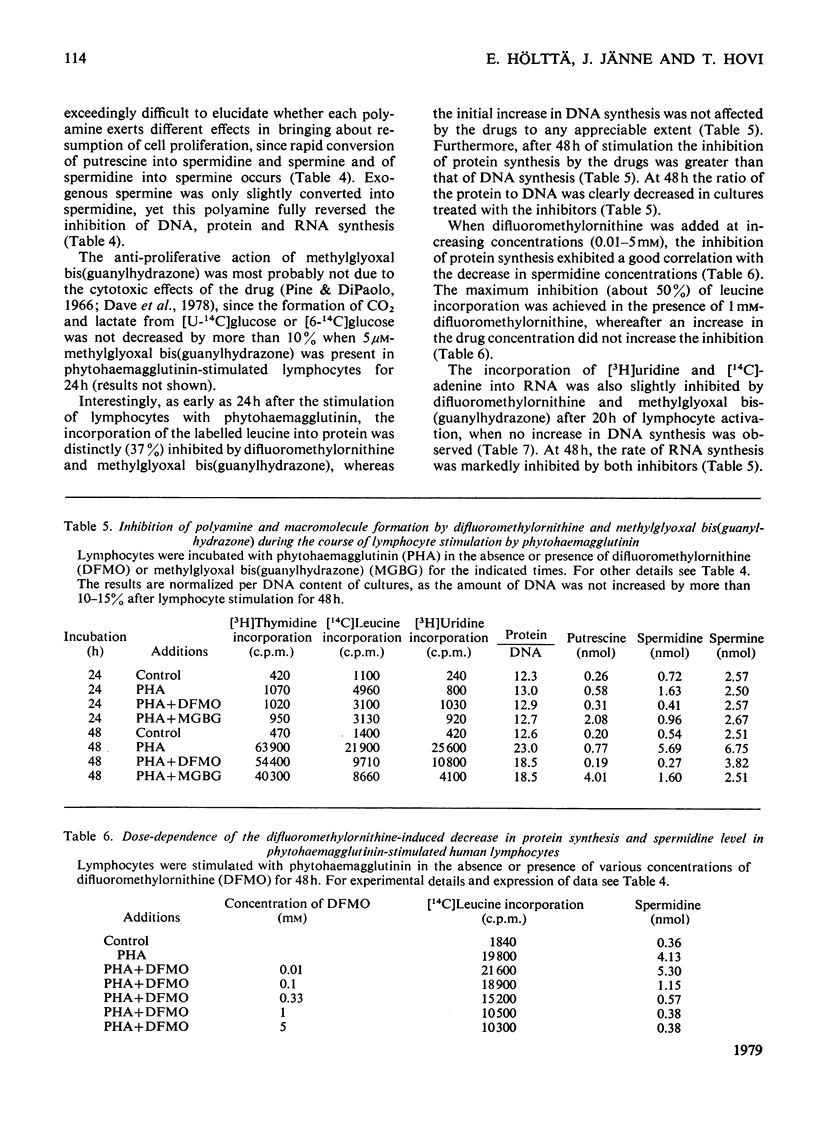
1. The activation of human peripheral blood lymphocytes by phytohaemagglutinin in vitro was accompanied by striking increases in the concentrations of the natural polyamines putrescine, spermidine and spermine. 2. The enhanced accumulation of polyamines could be almost totally abolished by dl-α-difluoromethylornithine, a newly discovered irreversible inhibitor of l-ornithine decarboxylase (EC 4.1.1.17), or by methylglyoxal bis(guanylhydrazone) {1,1′-[(methylethanediylidene)dinitrilo]diguanidine}, an inhibitor of S-adenosyl-l-methionine decarboxylase (EC 4.1.1.50). The inhibition of polyamine accumulation was associated with a marked suppression of DNA synthesis, which was partially or totally reversed by low concentrations of exogenous putrescine, spermidine, spermine and cadaverine and by higher concentrations of 1,3-diaminopropane. 3. In contrast with some earlier studies, we found that methylglyoxal bis(guanylhydrazone), at concentrations that were sufficient to prevent polyamine accumulation, also caused a clear inhibition of protein synthesis in the activated lymphocytes. Similar results were obtained with difluoromethylornithine. The decrease in protein synthesis caused by both compounds preceded the impairment of DNA synthesis. The inhibition of protein synthesis by difluoromethylornithine was fully reversed by exogenous putrescine, spermidine and spermine, and that caused by methylglyoxal bis(guanylhydrazone) by spermidine and spermine. In further support of the idea that the inhibition of protein synthesis by these compounds was related to the polyamine depletion, we found that difluoromethylornithine caused a dose-dependent decrease in the incorporation of [14C]leucine into lymphocyte proteins which closely correlated with the decreased concentrations of cellular spermidine. 4. Difluoromethylornithine and methylglyoxal bis(guanylhydrazone) also elicited a variable depression in the incorporation of [3H]uridine and [14C]adenine into total RNA. The apparent turnover of lymphocyte RNA remained essentially unchanged in spite of severe polyamine depletion brought about by difluoromethylornithine. 5. The present results, as well as confirming the anti-proliferative action of the inhibitors of polyamine biosynthesis, suggest that polyamine depletion may interfere with reactions at different levels of gene expression.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barlow S. D., Ord M. G. Thymidine transport in phytohaemagglutinin-stimulated pig lymphocytes. Biochem J. 1975 May;148(2):295–302. doi: 10.1042/bj1480295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyfuss G., Dvir R., Harell A., Chayen R. Determination of polyamines in urine. Clin Chim Acta. 1973 Nov 23;49(1):65–72. doi: 10.1016/0009-8981(73)90344-6. [DOI] [PubMed] [Google Scholar]
- Fillingame R. H., Jorstad C. M., Morris D. R. Increased cellular levels of spermidine or spermine are required for optimal DNA synthesis in lymphocytes activated by concanavalin A. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4042–4045. doi: 10.1073/pnas.72.10.4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillingame R. H., Morris D. R. Polyamine accumulation during lymphocyte transformation and its relation to the synthesis, processing, and accumulation of ribonucleic acid. Biochemistry. 1973 Oct 23;12(22):4479–4487. doi: 10.1021/bi00746a028. [DOI] [PubMed] [Google Scholar]
- Hausen P., Stein H., Peters H. On the systhesis of RNA in lymphocytes stimulated by phytohemagglutinin. The activity of deoxyribonucleoprotein-bound and soluble RNA polymerase. Eur J Biochem. 1969 Jul;9(4):542–549. doi: 10.1111/j.1432-1033.1969.tb00643.x. [DOI] [PubMed] [Google Scholar]
- Hauser H., Knippers R., Schäfer K. P. Increased rate of RNA-polyadenylation. An early response in Concanavalin A activated lymphocytes. Exp Cell Res. 1978 Jan;111(1):175–184. doi: 10.1016/0014-4827(78)90247-1. [DOI] [PubMed] [Google Scholar]
- Hoskins D. D., Patterson D. L. Metabolism of rhesus monkey spermatozoa. J Reprod Fertil. 1968 Jul;16(2):183–195. doi: 10.1530/jrf.0.0160183. [DOI] [PubMed] [Google Scholar]
- Hovi T., Allison A. C., Williams S. C. Proliferation of human peripheral blood lymphocytes induced by A23187, a streptomyces antibiotic. Exp Cell Res. 1976 Jan;97:92–100. doi: 10.1016/0014-4827(76)90658-3. [DOI] [PubMed] [Google Scholar]
- Jänne J., Pösö H., Raina A. Polyamines in rapid growth and cancer. Biochim Biophys Acta. 1978 Apr 6;473(3-4):241–293. doi: 10.1016/0304-419x(78)90015-x. [DOI] [PubMed] [Google Scholar]
- Kay J. E. Early effects of phytohaemagglutinin on lymphocyte RNA synthesis. Eur J Biochem. 1968 Apr 3;4(2):225–232. doi: 10.1111/j.1432-1033.1968.tb00198.x. [DOI] [PubMed] [Google Scholar]
- Kay J. E., Lindsay V. J. Polyamine synthesis during lymphocyte activation. Induction of ornithine decarboxylase and S-adenosyl methionine decarboxylase. Exp Cell Res. 1973 Mar 15;77(1):428–436. doi: 10.1016/0014-4827(73)90597-1. [DOI] [PubMed] [Google Scholar]
- Kay J. E., Pegg A. E. Effect of inhibition of spermidine formation on protein and nucleic acid synthesis during lymphocyte activation. FEBS Lett. 1973 Feb 1;29(3):301–304. doi: 10.1016/0014-5793(73)80044-4. [DOI] [PubMed] [Google Scholar]
- Krokan H., Eriksen A. DNA synthesis in HeLa cells and isolated nuclei after treatment with an inhibitor of spermidine synthesis, methyl glyoxal bis(guanylhydrazone). Eur J Biochem. 1977 Feb;72(3):501–508. doi: 10.1111/j.1432-1033.1977.tb11273.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MASTER R. W. POSSIBLE SYNTHESIS OF POLYRIBONUCLEOTIDES OF KNOWN BASE-TRIPLET SEQUENCES. Nature. 1965 Apr 3;206:93–93. doi: 10.1038/206093b0. [DOI] [PubMed] [Google Scholar]
- Mamont P. S., Duchesne M. C., Grove J., Bey P. Anti-proliferative properties of DL-alpha-difluoromethyl ornithine in cultured cells. A consequence of the irreversible inhibition of ornithine decarboxylase. Biochem Biophys Res Commun. 1978 Mar 15;81(1):58–66. doi: 10.1016/0006-291x(78)91630-3. [DOI] [PubMed] [Google Scholar]
- Morris D. R., Jorstad C. M., Seyfried C. E. Inhibition of the synthesis of polyamines and DNA in activated lymphocytes by a combination of alpha-methylornithine and methylglyoxal bis(guanylhydrazone). Cancer Res. 1977 Sep;37(9):3169–3172. [PubMed] [Google Scholar]
- Munch-Petersen B., Tyrsted G. Induction of thymidine kinases in phytohaemagglutinin-stimulated human lymphocytes. Biochim Biophys Acta. 1977 Oct 4;478(3):364–375. doi: 10.1016/0005-2787(77)90152-6. [DOI] [PubMed] [Google Scholar]
- Otani S., Matsui I., Morisawa S. Suppression of phytohemagglutinin-induction of thymidine uptake in guinea pig lymphocytes by methylglyoxal bis(guanylhydrazone) treatment. Biochim Biophys Acta. 1977 Oct 18;478(4):417–427. doi: 10.1016/0005-2787(77)90097-1. [DOI] [PubMed] [Google Scholar]
- Pine M. J., DiPaolo J. A. The antimitochondrial action of 2-choloro-4', 4"-bis(2-imidazolin-2-yl)terephthalanilide and methylglyoxal bis(guanylhydrazone). Cancer Res. 1966 Jan;26(1):18–25. [PubMed] [Google Scholar]
- Pösö H., Jänne J. Inhibition of polyamine accumulation and deoxyribonucleic acid synthesis in regenerating rat liver. Biochem J. 1976 Aug 15;158(2):485–488. doi: 10.1042/bj1580485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler N. Use of the dansyl reaction in biochemical analysis. Methods Biochem Anal. 1970;18:259–337. doi: 10.1002/9780470110362.ch5. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. 1,4-Diaminobutane (putrescine), spermidine, and spermine. Annu Rev Biochem. 1976;45:285–306. doi: 10.1146/annurev.bi.45.070176.001441. [DOI] [PubMed] [Google Scholar]
- Williams-Ashman H. G., Schenone A. Methyl glyoxal bis(guanylhydrazone) as a potent inhibitor of mammalian and yeast S-adenosylmethionine decarboxylases. Biochem Biophys Res Commun. 1972 Jan 14;46(1):288–295. doi: 10.1016/0006-291x(72)90661-4. [DOI] [PubMed] [Google Scholar]
- Young D. V., Srinivasan P. R. Regulation of macromolecular synthesis by putrescine in a conditional Escherichia coli putrescine auxotroph. J Bacteriol. 1972 Oct;112(1):30–39. doi: 10.1128/jb.112.1.30-39.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]


