Abstract
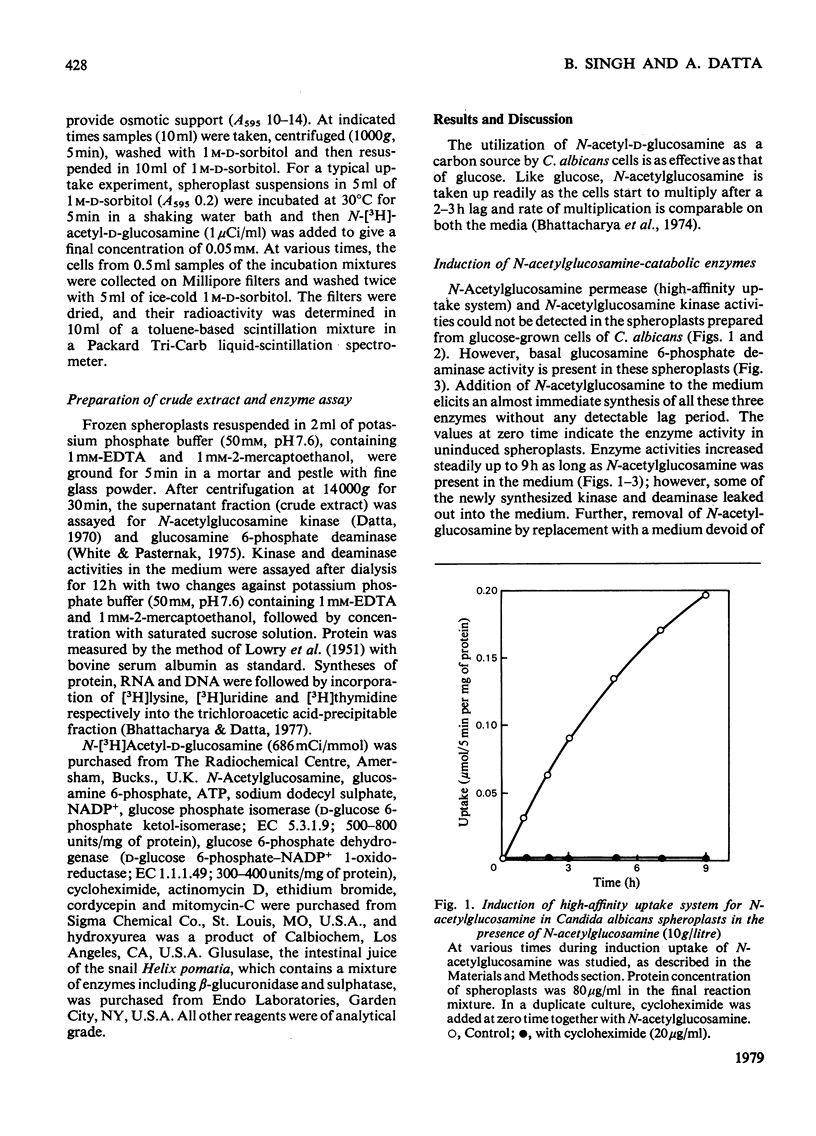
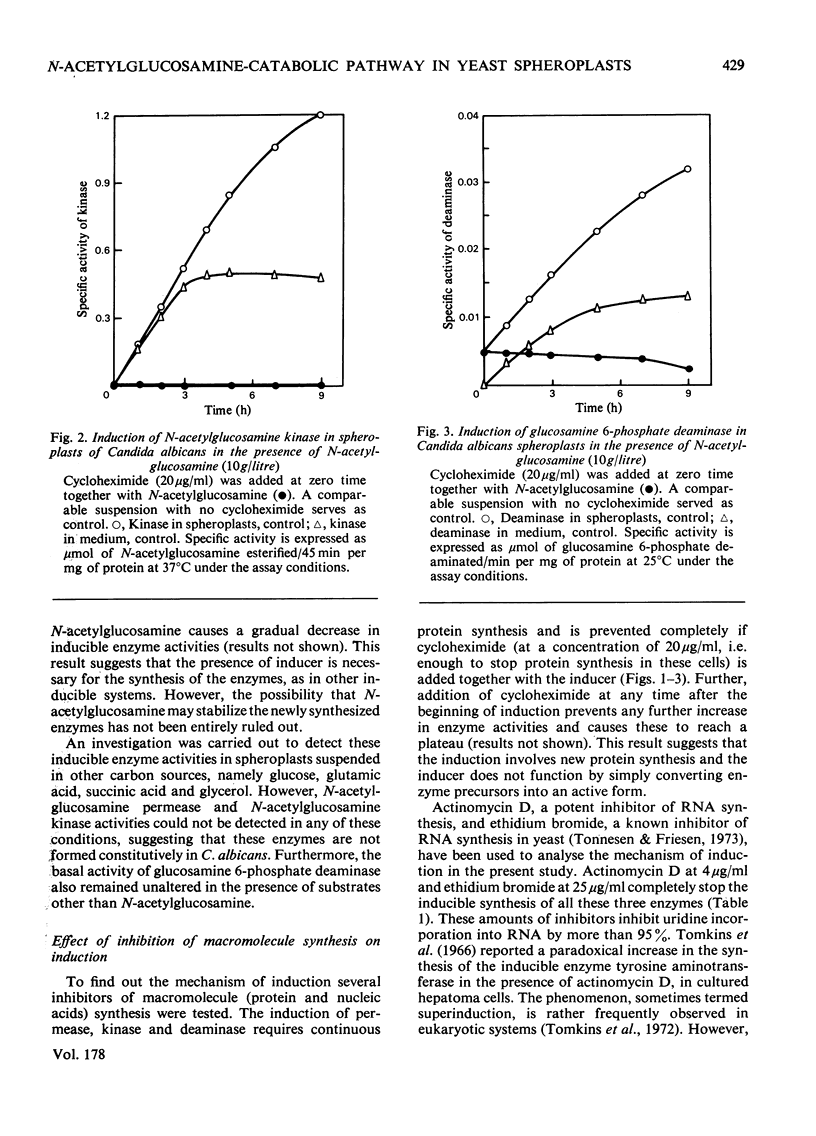
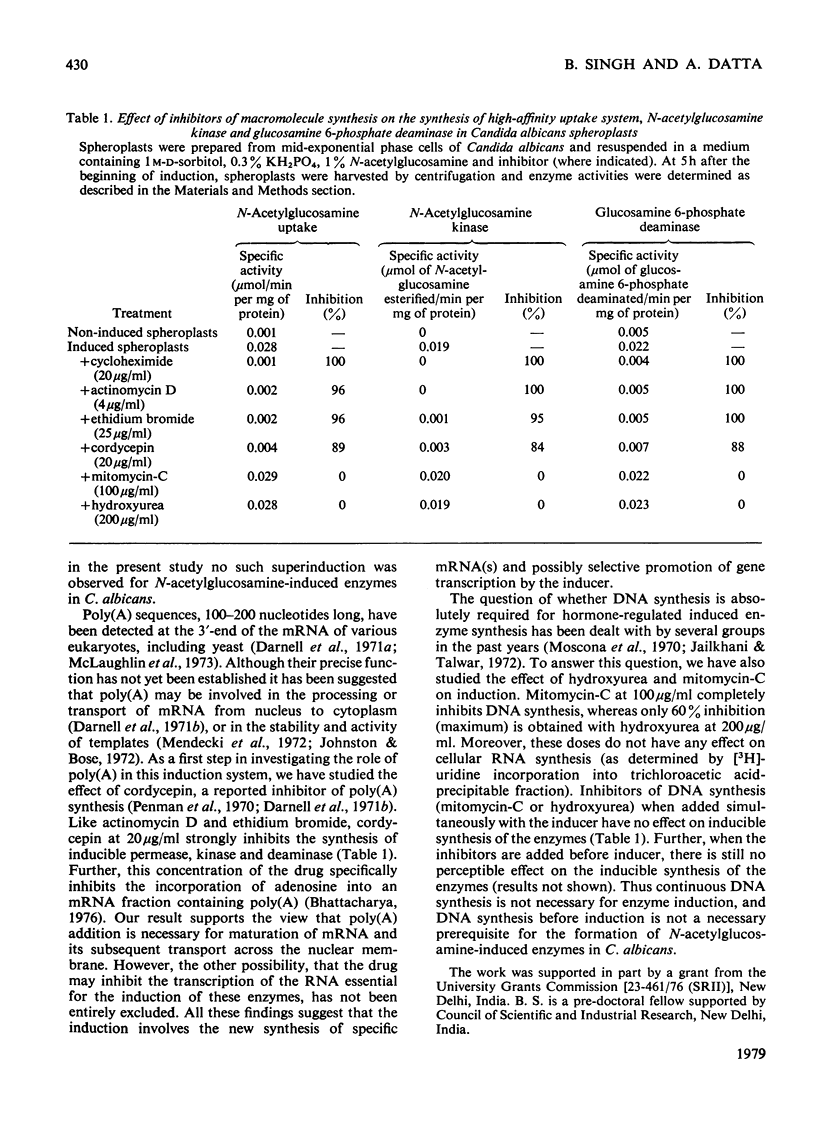
Synthesis of N-acetylglucosamine-catabolic enzymes, namely permease (high-affinity uptake system), kinase and deaminase was studied in the spheroplasts of the yeast Candida albicans. The presence of N-acetylglucosamine as inducer is essential for the induced synthesis of these enzymes in the spheroplasts, which were active for at least 8--9 h. However, some of the newly synthesized kinase and deaminase leaked out from the spheroplasts into the medium during induction. Experiments with inhibitors of RNA and protein synthesis indicate that the appearance of new enzyme activities is dependent on concomitant new protein synthesis and the inducer operates at a transcriptional level. However, inhibitors of DNA synthesis, e.g. mitomycin-C and hydroxyurea, had no effect on the synthesis of these enzymes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhattacharya A., Banerjee S., Datta A. Regulation of N-acetylglucosamine kinase synthesis in yeast. Biochim Biophys Acta. 1974 Dec 20;374(3):384–391. doi: 10.1016/0005-2787(74)90259-7. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A., Datta A. Effect of cyclic AMP on RNA and protein synthesis in Candida albicans. Biochem Biophys Res Commun. 1977 Aug 22;77(4):1483–1444. doi: 10.1016/s0006-291x(77)80140-x. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Philipson L., Wall R., Adesnik M. Polyadenylic acid sequences: role in conversion of nuclear RNA into messenger RNA. Science. 1971 Oct 29;174(4008):507–510. doi: 10.1126/science.174.4008.507. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Wall R., Tushinski R. J. An adenylic acid-rich sequence in messenger RNA of HeLa cells and its possible relationship to reiterated sites in DNA. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1321–1325. doi: 10.1073/pnas.68.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A. Studies on hog spleen N-acetylglucosamine kinase. I. Purification and properties of N-acetylglucosamine kinase. Biochim Biophys Acta. 1970 Oct 14;220(1):51–60. doi: 10.1016/0005-2744(70)90228-7. [DOI] [PubMed] [Google Scholar]
- Holmes R. P., Russell R. R. Mutations affecting amino sugar metabolism in Escherichia coli K-12. J Bacteriol. 1972 Jul;111(1):290–291. doi: 10.1128/jb.111.1.290-291.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jailkhani B. L., Talwar G. P. Induction of phosvitin by oestradiol in rooster liver needs DNA synthesis. Nat New Biol. 1972 Oct 25;239(95):240–241. doi: 10.1038/newbio239240a0. [DOI] [PubMed] [Google Scholar]
- Johnston R. E., Bose H. R. Correlation of messenger RNA function with adenylate-rich segments in the genomes of single-stranded RNA viruses. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1514–1516. doi: 10.1073/pnas.69.6.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McLaughlin C. S., Warner J. R., Edmonds M., Nakazato H., Vaughan M. H. Polyadenylic acid sequences in yeast messenger ribonucleic acid. J Biol Chem. 1973 Feb 25;248(4):1466–1471. [PubMed] [Google Scholar]
- Mendecki J., Lee S. Y., Brawerman G. Characteristics of the polyadenylic acid segment associated with messenger ribonucleic acid in mouse sarcoma 180 ascites cells. Biochemistry. 1972 Feb 29;11(5):792–798. doi: 10.1021/bi00755a018. [DOI] [PubMed] [Google Scholar]
- Moscona A. A., Moscona M., Jones R. E. Induction of glutamine synthetase in embryonic neural retina in vitro by inhibitors of macromolecular synthesis. Biochem Biophys Res Commun. 1970 Jun 5;39(5):943–949. doi: 10.1016/0006-291x(70)90415-8. [DOI] [PubMed] [Google Scholar]
- Penman S., Rosbash M., Penman M. Messenger and heterogeneous nuclear RNA in HeLa cells: differential inhibition by cordycepin. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1878–1885. doi: 10.1073/pnas.67.4.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomkins G. M., Levinson B. B., Baxter J. D., Dethlefsen L. Further evidence for posttranscriptional control of inducible tyrosine aminotransferase synthesis in cultured hepatoma cells. Nat New Biol. 1972 Sep 6;239(88):9–14. doi: 10.1038/newbio239009a0. [DOI] [PubMed] [Google Scholar]
- Tonnesen T., Friesen J. D. Inhibitors of ribonucleic acid synthesis in Saccharomyces cerevisiae: decay rate of messenger ribonucleic acid. J Bacteriol. 1973 Sep;115(3):889–896. doi: 10.1128/jb.115.3.889-896.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. J. Control of amino sugar metabolism in Escherichia coli and isolation of mutants unable to degrade amino sugars. Biochem J. 1968 Feb;106(4):847–858. doi: 10.1042/bj1060847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. J., Pasternak C. A. N-acetylglucosamine-6-phosphate deacetylase and glucosamine-6-phosphate deaminase from Escherichia coli. Methods Enzymol. 1975;41:497–502. doi: 10.1016/s0076-6879(75)41105-3. [DOI] [PubMed] [Google Scholar]
- White R. J., Pasternak C. A. The purification and properties of N-acetylglucosamine 6-phosphate deacetylase from Escherichia coli. Biochem J. 1967 Oct;105(1):121–125. doi: 10.1042/bj1050121. [DOI] [PMC free article] [PubMed] [Google Scholar]


