Abstract
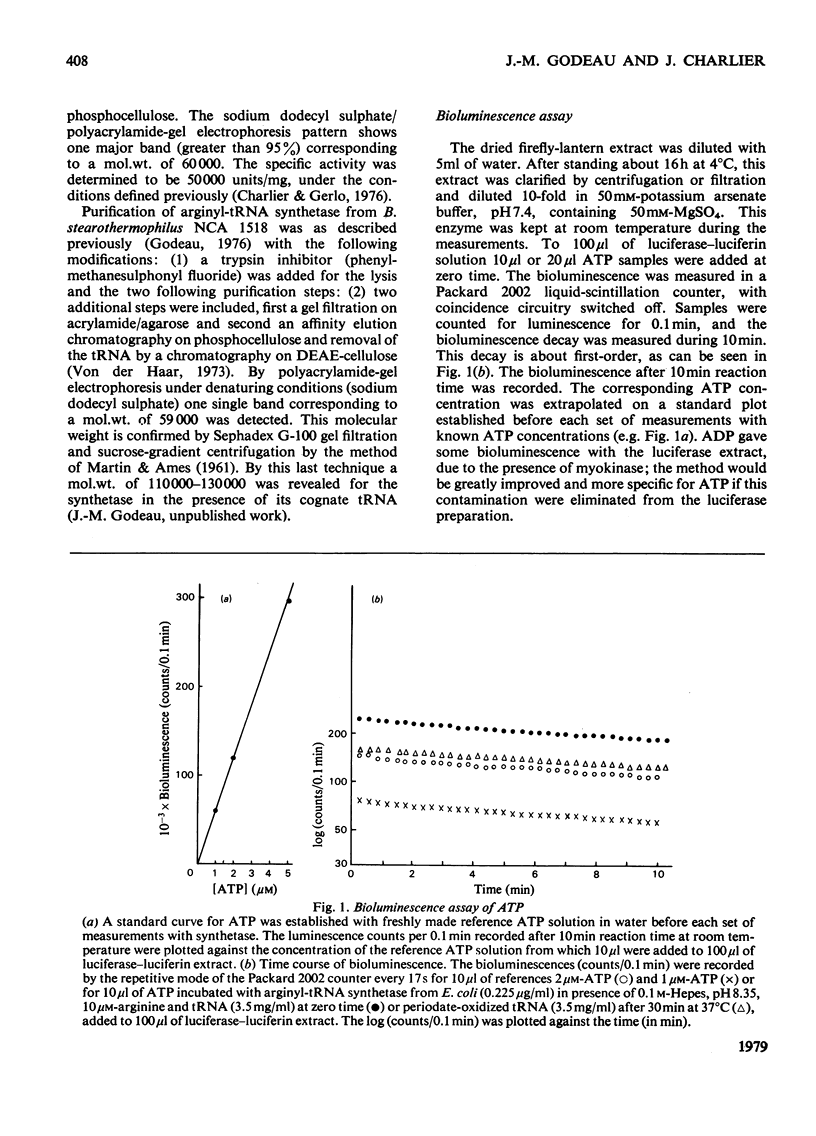
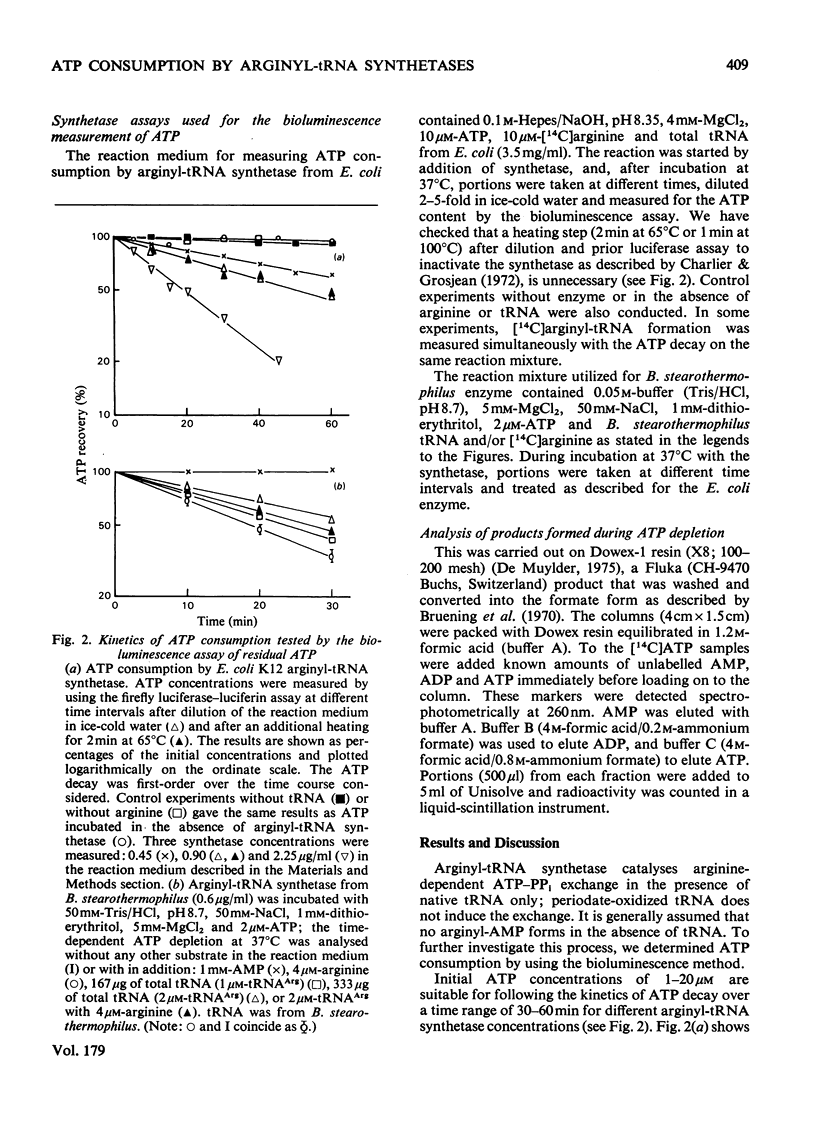
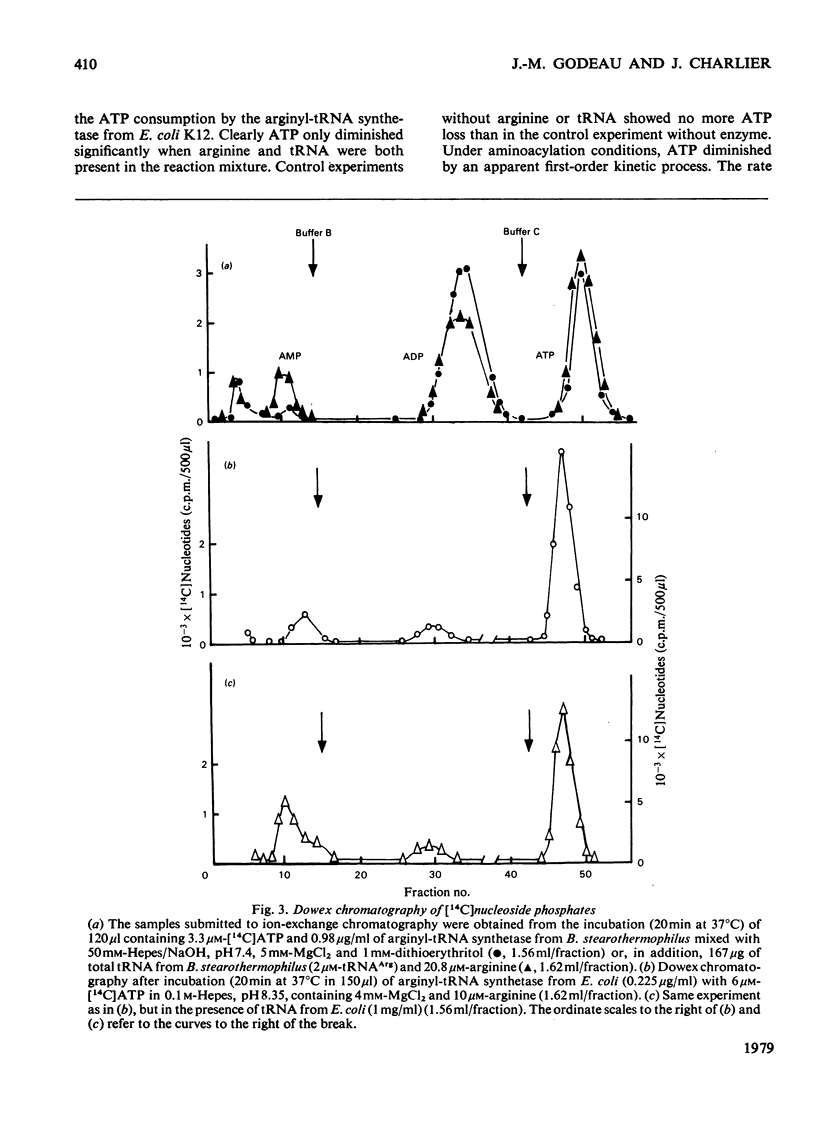
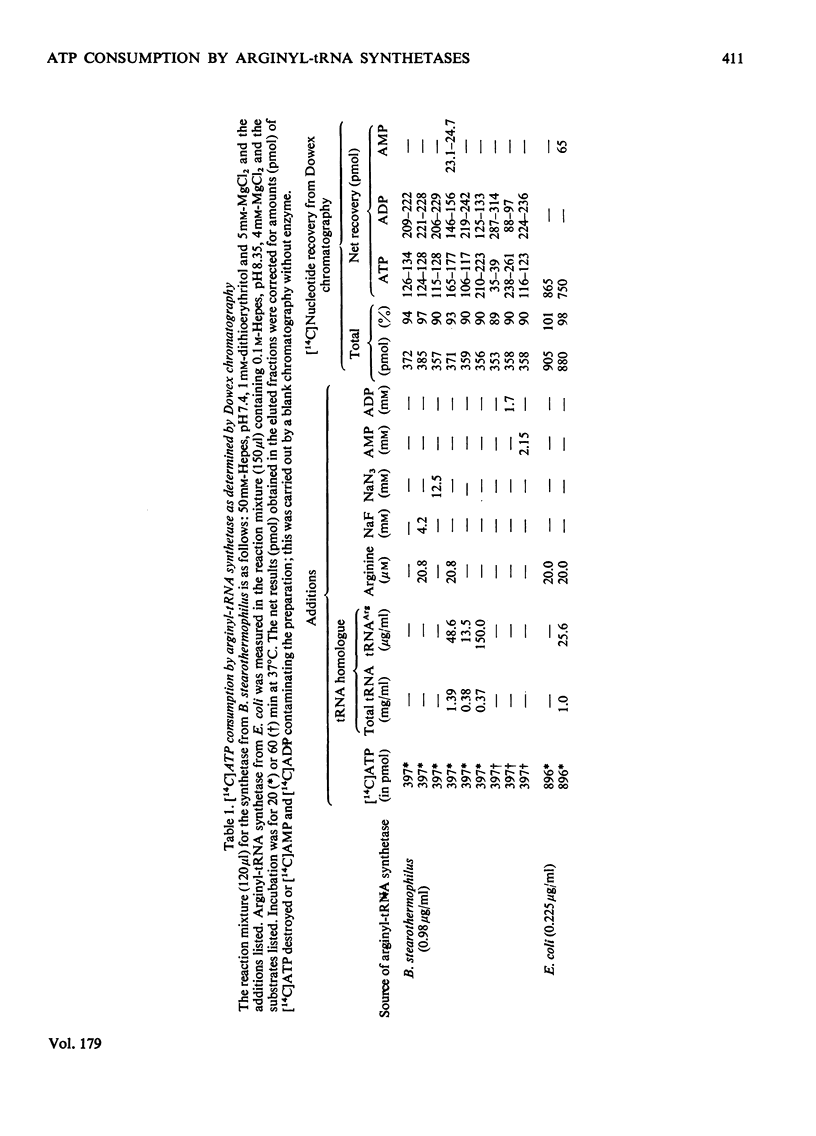
ATP consumption by arginyl-tRNA synthetases from Escherichia coli and Bacillus stearothermophilus has been investigated by the firefly luciferin--luciferase assay. Arginyl-tRNA synthetase from E. coli utilizes ATP only for aminocylation of tRNA with a 1:1 stoicheiometry. In contrast, we have shown an adenosine triphosphatase activity of arginyl-tRNA synthetase from B. stearothermophilus in the absence of tRNAArg. Dowex chromatography revealed the formation of ADP by the thermophile enzyme; under aminoacylation conditions, AMP was also formed in amounts stoicheiometric with arginyl-tRNA formation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRUBAKER L. H., MCCORQUODALE D. J. THE PREPARATION OF AMINO ACID-TRANSFER RIBONUCLEIC ACID FROM ESCHERICHIA COLI BY DIRECT PHENOL EXTRACTION OF INTACT CELLS. Biochim Biophys Acta. 1963 Sep 17;76:48–53. [PubMed] [Google Scholar]
- Charlier J., Gerlo E. Arginyl-tRNA synthetase from Escherichia coli. Influence of arginine biosynthetic precursors on the charging of arginine-acceptor tRNA with [14C]arginine. Eur J Biochem. 1976 Nov 1;70(1):137–145. doi: 10.1111/j.1432-1033.1976.tb10964.x. [DOI] [PubMed] [Google Scholar]
- Charlier J., Grosjean H. Isoleucyl-transfer ribonucleic acid synthetase from Bacillus stearothermophilus. I. Properties of the enzyme. Eur J Biochem. 1972 Jan 31;25(1):163–174. doi: 10.1111/j.1432-1033.1972.tb01681.x. [DOI] [PubMed] [Google Scholar]
- Gillam I., Blew D., Warrington R. C., von Tigerstrom M., Tener G. M. A general procedure for the isolation of specific transfer ribonucleic acids. Biochemistry. 1968 Oct;7(10):3459–3468. doi: 10.1021/bi00850a022. [DOI] [PubMed] [Google Scholar]
- Godeau J. M. Arginyl-tRNA synthetase from Bacillus stearothermophilus: subunit structure of enzyme. FEBS Lett. 1976 Feb 15;62(2):190–193. doi: 10.1016/0014-5793(76)80050-6. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- NORRIS A. T., BERG P. MECHANISM OF AMINOACYL RNA SYNTHESIS: STUDIES WITH ISOLATED AMINOACYL ADENYLATE COMPLEXES OF ISOLEUCYL RNA SYNTHETASE. Proc Natl Acad Sci U S A. 1964 Aug;52:330–337. doi: 10.1073/pnas.52.2.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEPHENSON M. L., ZAMECNIK P. C. Purification of valine transfer ribonucleic acid by combined chromatographic and chemical procedures. Proc Natl Acad Sci U S A. 1961 Oct 15;47:1627–1635. doi: 10.1073/pnas.47.10.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarus M., Berg P. On the properties and utility of a membrane filter assay in the study of isoleucyl-tRNA synthetase. Anal Biochem. 1970 Jun;35(2):450–465. doi: 10.1016/0003-2697(70)90207-1. [DOI] [PubMed] [Google Scholar]
- von der Haar F. Affinity elution as a purification method for aminoacyl-tRNA synthetases. Eur J Biochem. 1973 Apr 2;34(1):84–90. doi: 10.1111/j.1432-1033.1973.tb02731.x. [DOI] [PubMed] [Google Scholar]


