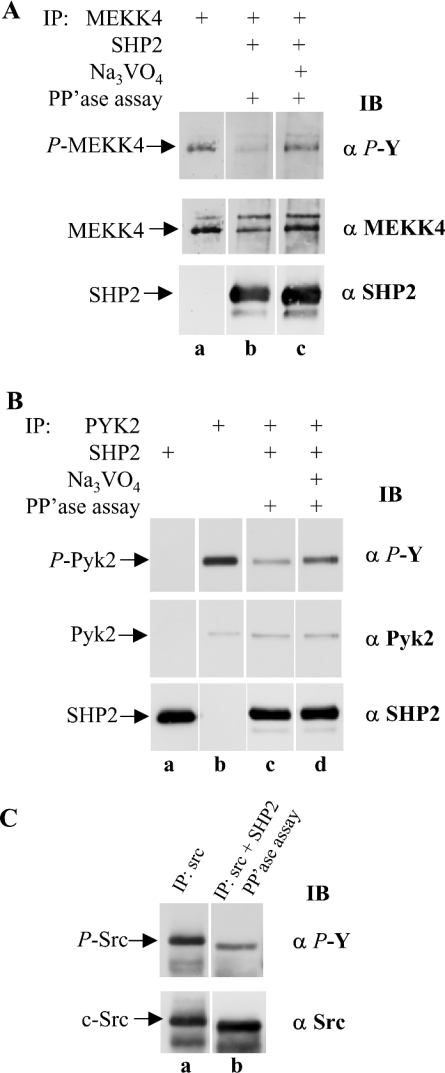
Figure 8. SHP2 recognizes MEKK4 from HaCaT cells as substrate.
(A) MEKK4 and SHP2 were immunoprecipitated (IP) separately from HaCaT cells treated for 25 min with IFNγ. To assay SHP2 phosphatase activity, MEKK4 and SHP2 immunoprecipitates were combined and incubated under phosphatase (PP'ase) assay conditions (lanes b and c). SHP2 activity was blocked by adding 2 mM Na3VO4 to the reaction mixture (lane c). SHP2 activity was monitored by phosphotyrosine (α P-Y) immunoblot (IB) analysis. The presence of SHP2 resulted in decreased tyrosylphosphate on MEKK4. For a loading control, the membrane was reprobed against MEKK4 (α MEKK4) and SHP2 (α SHP2). Similarly, (B) immunoprecipitation of Pyk2, and combined immunoprecipitation of Pyk2 and SHP2, with immunoblotting with anti-phosphotyrosine (α P-Y), anti-Pyk2 (α Pyk2) or anti-SHP2 (α SHP2) antibodies; (C) immunoprecipitation of Src, and combined immunoprecipitation of Src and SHP2 from HaCaT cells treated for 2 min with IFNγ and subjected to phosphatase assay conditions as described in (A) and were then immunoblotted for phosphotyrosine (α P-Y) or Src (α Src). Active SHP2 dephosphorylates Pyk2 and c-Src as compared with Na3VO4-inhibited SHP2.