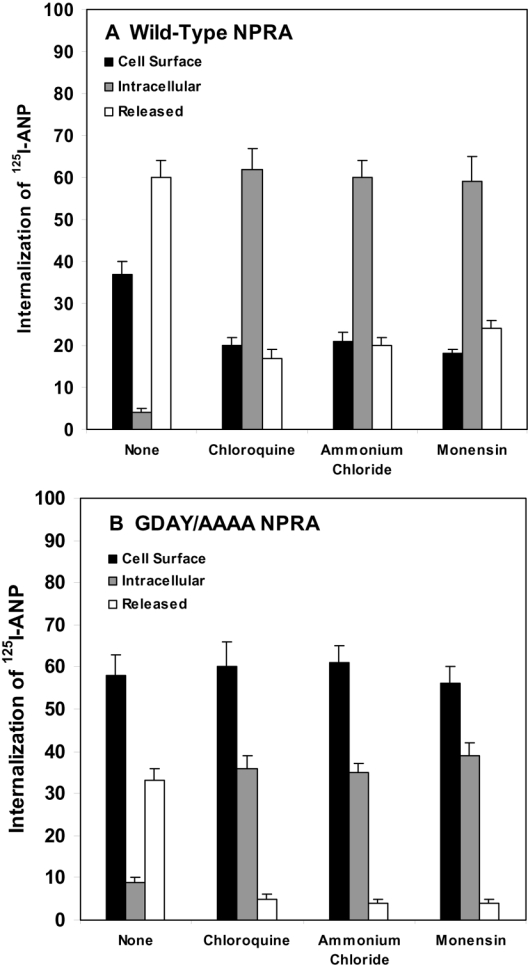
Figure 6. Quantitative analyses of cell-surface-associated, internalized and released 125I-ANP radioactivity after treatment with lysosomotropic agents in HEK-293 cells expressing wild-type or GDAY/AAAA mutant NPRA.
Confluent HEK-293 cells expressing either (A) wild-type or (B) mutant receptors were washed with assay medium and preincubated in 2 ml of fresh assay medium with the indicated lysosomotropic agents: chloroquine (200 μM), ammonium chloride (10 mM) and monensin (50 μM), at 37 °C for 60 min. The cells were then washed with assay medium and incubated with 125I-ANP in 2 ml of fresh assay medium for 60 min at 4 °C as described in the Materials and methods section. The treatment with lysosomotropic agents was maintained throughout the ligand-binding and internalization periods of the experiment. After the binding was completed, cells were washed four times with the assay medium and then placed in 2 ml of fresh medium at 37 °C. After 10 min of internalization and incubation, culture dishes were removed from the medium at 37 °C and placed at 4 °C. The medium was collected and each dish was treated with 2 ml of cold glycine acidic buffer (pH 3.8) for 2 min at 4 °C. After collecting the acid eluate, cells were dissolved in 0.5 M NaOH. 125I-ANP radioactivity in the acid eluate, cell extract and culture medium was counted to determine the cell-surface-associated, internalized and released 125I-ANP radioactivity respectively. The results represent the means±S.E.M. for three independent determinations with triplicate dishes in each experiment.