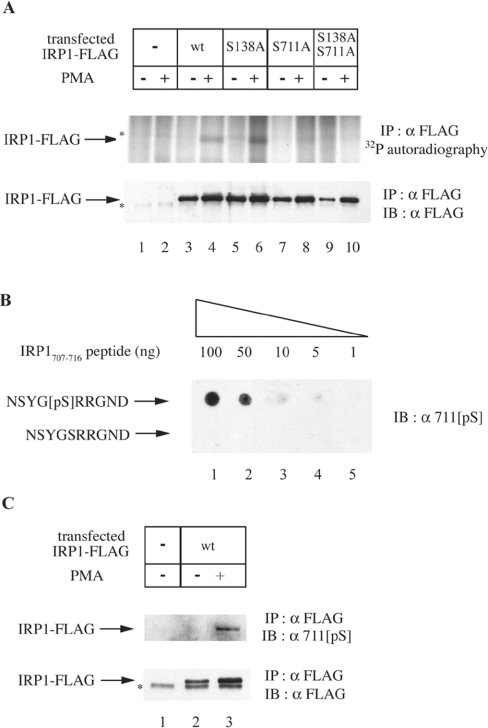
Figure 1. Ser-711 is the site of IRP1 phosphorylation in cells treated with PMA.
(A) HEK-293 cells were transiently transfected with constructs encoding FLAG-tagged wild-type (wt) or mutant (S138A, S711A or S138A/S711A) IRP1. Parent and transfected cells were metabolically labelled with [32P]orthophosphate. After 30 min, 0.2 μM PMA (dissolved in DMSO) or solvent alone were added in the radioactive medium, and the treatment was continued for 90 min. To assess the phosphorylation status of transfected wild-type IRP1, IRP1S138A, IRP1S711A and IRP1S138A/S711A, cytoplasmic extracts (1000 μg) were subjected to quantitative immunoprecipitation (IP) with a FLAG antibody (8.8 μg). The immunoprecipitated material was analysed by SDS/PAGE (8% polyacrylamide). Phosphorylated proteins were visualized by autoradiography (upper panel) and the recovery of transfected IRP1 was analysed by immunoblotting (IB) with 1:1000 diluted FLAG antibody (lower panel). (B) The indicated amounts of NSYG[pS]RRGND and NSYGSRRGND peptides were spotted on a nitrocellulose filter and analysed by IB with 1:1000 diluted 711[pS], a phospho-specific antibody raised against NSYG[pS]RRGND. (C) HEK-293 cells were transiently transfected with a construct encoding FLAG-tagged wild-type IRP1 and treated with 0.2 μM PMA or DMSO alone for 90 min. To assess phosphorylation of IRP1 at Ser-711, cytoplasmic extracts (1000 μg) were subjected to quantitative IP with the FLAG antibody (8.8 μg) and subsequently analysed by IB with 1:1000 diluted 711[pS] antibody (upper panel). The recovery of transfected IRP1 was analysed by immunoblotting with the FLAG antibody (lower panel). The asterisks denote non-specific bands.