Abstract
Recent studies by Moneer and Taylor [(2002) Biochem. J. 362, 13–21] have proposed a reciprocal regulation of two Ca2+-entry pathways by AVP ([Arg8]-vasopressin) in A7r5 vascular smooth-amuscle cells. Their model proposes that AVP inhibits CCE (capacitative Ca2+ entry) and predicts a rebound of CCE after the removal of AVP. In the present study, we used whole-cell perforated patch-clamp techniques to measure ISOC (store-operated current) corresponding to CCE in A7r5 cells. When 100 nM AVP is present, it activates ISOC with no apparent rebound on removal of AVP. ISOC activated by thapsigargin or cyclopiazonic acid was not inhibited by 100 nM AVP. We also used fura 2 fluorescence techniques to re-examine the model of Moneer and Taylor, specifically focusing on the proposed inhibition of CCE by AVP. We find that 100 nM AVP activates capacitative Mn2+ entry and does not inhibit thapsigargin- or cyclopiazonic acid-activated Mn2+ entry. Moreover, Ca2+ entry after depletion of intracellular Ca2+ stores is enhanced by AVP and we detect no rebound of Ca2+ or Mn2+ entry after AVP removal. On the basis of these findings, we conclude that AVP does not inhibit CCE in A7r5 cells.
Keywords: A7r5 cells, calcium entry, fura 2, store-operated current, vascular smooth muscle, vasopressin
Abbreviations: [Ca2+]i, cytosolic free Ca2+ concentration; AVP, [Arg8]-vasopressin; BAPTA, bis(2-amino-5-bromophenoxy)ethane-N,N,N′,N′-tetra-acetic acid; CCE, capacitative Ca2+ entry; CPA, cyclopiazonic acid; NCCE, non-capacitative Ca2+ entry; fura 2/AM, fura 2 acetoxymethyl ester; NMDG, N-methyl-D-glucamine
INTRODUCTION
Non-selective cation channels have been implicated in vasoconstrictor actions as a mechanism for regulating Ca2+ entry in vascular smooth-muscle cells. Ca2+ entering the cytosol through these channels may directly activate cell contraction, and the inward current carried by Ca2+ and Na+ may contribute to membrane depolarization for the activation of Ca2+ entry through L-type voltage-sensitive Ca2+ channels. We have demonstrated previously that AVP ([Arg8]-vasopressin) elicits distinct Ca2+ signalling patterns depending on its concentration [1,2]. Ca2+ spiking involving L-type voltage-sensitive Ca2+ channels predominates at the low concentrations of AVP found in the systemic circulation [2]. The roles of non-selective cation channels in these effects of AVP are yet to be examined. It will be important to elucidate the ionic mechanisms governing AVP-stimulated Ca2+-entry pathways to understand further their physiological functions.
Ca2+ entry, presumably through non-selective cation channels, has been studied in A7r5 vascular smooth-muscle cells using Ca2+-sensitive fluorescent indicators in cells treated with nimodipine or verapamil to exclude contributions of L-type Ca2+ channels [1,3–6]. Byron and Taylor [1] identified two distinct pathways for Ca2+ entry activated by AVP in A7r5 cells: a CCE (capacitative Ca2+-entry) pathway activated by the depletion of intracellular Ca2+ stores and an NCCE (non-capacitative Ca2+-entry) pathway activated specifically by AVP and with differing ionic permeabilities when compared with CCE. Recent studies by Moneer et al. [3,6] have suggested that AVP activates the NCCE pathway while simultaneously inhibiting the CCE pathway.
In the present study, we have examined the electrophysiological correlation of CCE by studying the store-operated current ISOC and its regulation by AVP. We find that ISOC is activated by AVP in a manner that is not consistent with the reciprocal regulation proposed by Moneer and Taylor [3]. It should be noted that several results of Moneer et al. [3,6] differ from or directly contradict earlier results from the same laboratory. For example: (i) Byron and Taylor [1] demonstrated that AVP had no effect on capacitative Mn2+ entry, but Moneer and Taylor [3] reported that AVP inhibits capacitative Mn2+ entry by as much as 90%; (ii) Broad et al. [4] reported that arachidonic acid had no effect on capacitative Mn2+ entry, whereas Moneer and Taylor [3] found that arachidonic acid inhibits CCE; (iii) Byron and Taylor [1] demonstrated that [Ca2+]i responses to [AVP]≥50 nM exhibited little plateau phase, whereas a pronounced and sustained increase of [Ca2+]i (∼300 nM above resting levels) is apparent in the results of Moneer and Taylor [3]; (iv) Byron and Taylor [1] found, using both cell populations and single cells, that AVP enhances Ba2+ entry beyond that stimulated by maximal store depletion, but Moneer and Taylor [3] reported that capacitative Ba2+ entry was decreased by AVP.
Because of the above-noted contradictions in the previous studies and because our electrophysiological results did not fit the reciprocal regulation model, we used fura 2 fluorescence methods to re-examine the CCE pathway and its regulation in A7r5 cells. Our present findings are consistent with the original findings of Byron and Taylor [1] and also support their conclusions that AVP directly activates an NCCE pathway that is relatively impermeable to Mn2+ and that AVP does not inhibit CCE [1]. The differences between the results of Moneer et al. [3,6] and our present and previous findings are discussed.
EXPERIMENTAL
A7r5 cell culture
A7r5 cells were propagated from low-passage stocks used in the studies of Byron and Taylor [1,7] and cultured as described previously [8]. Cells were subcultured on to rectangular (9 mm×22 mm, no. 11/2) glass coverslips or plastic tissue culture flasks (Corning, Acton, MA, U.S.A.). For fura 2 fluorescence measurements, confluent cell monolayers grown on glass coverslips were used 2–5 days after plating. For electrophysiological recordings, cells grown on plastic were trypsinized (0.25% trypsin and 1% EDTA) and replated on 12 mm round glass coverslips. Cells were allowed to adhere for approx. 15 min and then transferred on to external solution for 30 min to 2 h before recording.
Patch-clamp
Whole-cell perforated- or ruptured-patch configurations were used to measure membrane currents under voltage-clamp conditions in single A7r5 cells. All experiments were performed at room temperature (23–25 °C) with continuous perfusion of external solution (perfusion rates of 1 ml/min; ∼0.7 ml chamber volume).
The external solution for recording ISOC contained (in mM): 100 NMDG (N-methyl-D-glucamine) aspartate, 20 calcium aspartate, 1 MgCl2 and 10 Hepes (pH 7.3). Internal (pipette) solution contained (in mM): 90 caesium aspartate, 20 CsCl, 4.6 MgCl2, 5 Hepes, 10 BAPTA-Cs4 [where BAPTA stands for bis(2-amino-5-bromophenoxy)ethane-N,N,N′,N′-tetra-acetic acid; pH 7.2]. With no Ca2+ added, but assuming possible contamination from impure chemicals resulting in up to 10 μM total Ca2+, we estimate the concentration of free Ca2+ in the internal solution to be less than 0.4 nM (calculated with WINMAXC software).
The conditions used were intended for maximal isolation of ISOC from other currents, including another vasopressin-activated non-selective cation current, which was undetectably small in this external solution (results not shown). Cl− currents were minimized by the use of an impermeant anion, aspartate (Asp−). In addition, we conducted preliminary experiments (results not shown) to ensure that the inward current we measured was not carried by Cl−: first, replacing 40 mM external Asp− with 40 mM Cl− did not affect the current–voltage relationship (it had the same inward rectification, current density and reversal potential as ISOC that developed when Asp− was the only anion in the external solution); secondly, replacement of all external Asp− with Cl− after ISOC activation did not affect current densities or the reversal potential.
A well-accepted characteristic of ISOC is that it is inhibited by lanthanides such as Gd3+. CCE in A7r5 cells is also reportedly inhibited by low concentrations of Gd3+ (<5 μM [3,4,6]). To demonstrate the Gd3+ sensitivity of ISOC in A7r5 cells, we added 100 μM GdCl3 to the external solution at the end of the experiment (e.g. Figures 1G, 2A and 2B). We estimate that the final concentration of free Gd3+ is in the 1–5 μM range (calculated with WINMAXC software using Gd-aspartate binding constants kindly provided by C. Patton, Stanford University). Inhibition of the inward current by micromolar free Gd3+ is consistent with its identification as ISOC and also additionally distinguishes it from Cl− currents, which are generally lanthanide-insensitive [9,10].
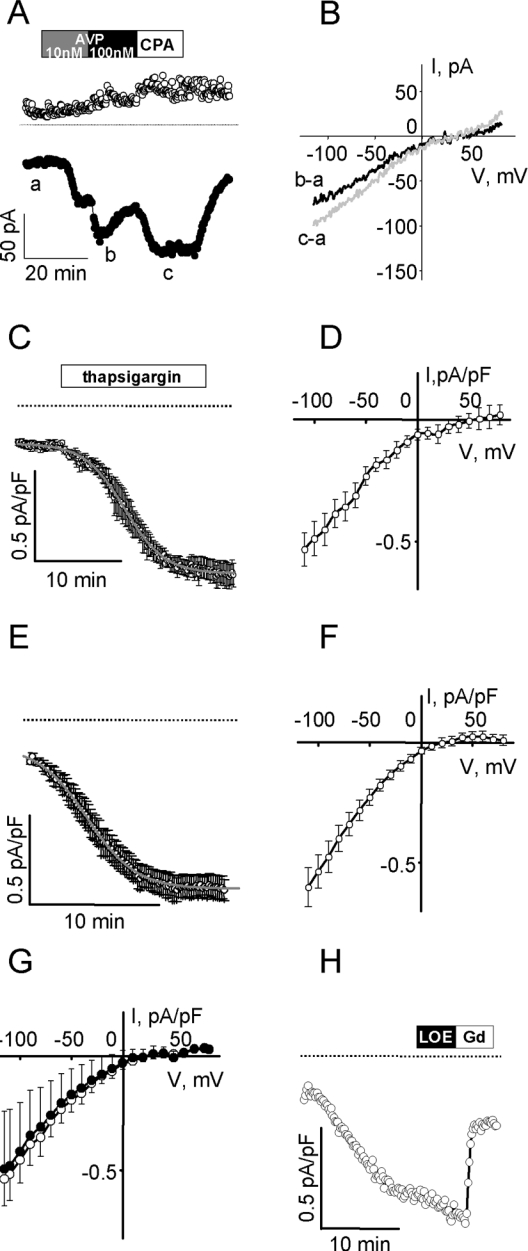
Figure 1. ISOC in A7r5 vascular smooth-muscle cells.
(A) Representative measurements of ISOC development in an A7r5 cell with 171.5 pF capacitance (C) during sequential application of 1 nM AVP (15 min, grey box), 100 nM AVP (15 min, black box) and 10 μM CPA (15 min, white box) followed by washout of CPA. Drugs were applied after stable recording of the resting current for 5 min. Current was recorded with a ramp voltage protocol (from +85 mV to −115 mV) from −15 mV holding potential every 10 s. Inward currents measured at −115 mV (●) and outward current measured at +85 mV (○) are presented. The dotted line indicates 0 current level. (B) Current–voltage relationships of AVP-activated (black line) and CPA-activated (grey line) ISOC recorded with the ramp voltage protocol at the time points indicated on (A) after subtraction of the resting current. Results are representative of at least three similar experiments. (C, D) Mean of perforated patch recordings (n=3) of inwardly rectifying current activated as a result of passive emptying of Ca2+ stores with 1 μM thapsigargin (irreversible inhibitor of sarcoplasmic reticulum Ca2+-ATPase) and the corresponding I–V curve of Gd3+-sensitive portion of current respectively. Time to half-maximal activation was 12.39±0.04 min. (E, F) The mean inwardly rectifying current that developed during dialysis of cells with 10 mM BAPTA (ruptured patch recordings, n=12) and the corresponding I–V curve. Time to half-maximal activation was 4.92±0.05 min. (G) Switching external monovalent cations do not affect maximal ISOC (activated by dialysis with 10 mM BAPTA). Mean I–V curves recorded in 20 mM Ca2++100 mM NMDG+ (○, n=4) and 20 mM Ca2++100 mM Na+ external solution (●, n=4). (H) ISOC activated by BAPTA dialysis was insensitive to 30 μM LOE 908, but was inhibited by the addition of 100 μM GdCl3 (C=174.5 pF, representative of four experiments).
Figure 2. Effects of AVP on ISOC in A7r5 vascular smooth-muscle cells.
(A) Time course of ISOC development was measured as described above; inward currents at −115 mV are presented as means±S.E.M. (n=5; capacitances in the range 45.5–159 pF). Boxes indicate application of 100 nM AVP and 100 μM GdCl3. Dotted lines indicate 0 current level. (B) AVP (100 nM) did not inhibit ISOC induced by passive depletion of Ca2+ store with 1 μM thapsigargin. Inward currents measured at −115 mV are presented as means±S.E.M. (n=4; capacitances in the range 219–432 pF). Boxes indicate application of 1 μM thapsigargin, 100 nM AVP and 100 μM GdCl3.
Nimodipine (0.5 μM) was present in all external solutions to inhibit L-type Ca2+ channels. Spermine chloride (100 μM) was also included to block the Mg2+-inhibitable current IMIC [11]. Osmolality of all solutions was adjusted to 270 mosmol/l with D-glucose. For membrane patch perforation, 200 μg/ml amphotericin B in the internal solution was used.
Experiments in whole-cell perforated patch configuration were started with series resistance Rs<30 MΩ; cells with an abrupt decrease in Rs were discarded. Recording of ISOC in ruptured patch configuration was started 30 s after break-in. Voltage-clamp command potentials were generated using an Axopatch 200B amplifier under the control of PCLAMP8 software. Time courses of ISOC development during exposure to 1 and 100 nM AVP, 10 μM CPA (cyclopiazonic acid), 1 μM thapsigargin or cell dialysis with 10 mM internal BAPTA were recorded every 10 s by a ramp voltage protocol (from +85 to −115 mV starting from a −15 mV holding potential). Whole-cell currents were digitized at 10 kHz, filtered at 5 kHz and analysed off-line. Currents were normalized to membrane capacitance. Whole-cell capacitance was compensated. Liquid junction potentials were calculated using junction potential calculator provided by PCLAMP8 software and subtracted off-line. Results are presented as means±S.E.M.
[Ca2+]i measurements
A7r5 cells were grown to confluence on glass coverslips. Coverslips were washed twice with control medium (135 mM NaCl, 5.9 mM KCl, 1.5 mM CaCl2, 1.2 mM MgCl2, 11.5 mM glucose and 11.6 mM Hepes, pH 7.3) and then incubated in the same medium with 4 μM fura 2/AM (fura 2 acetoxymethyl ester), 0.1% BSA and 0.02% Pluronic F127 detergent for 90–120 min at 25–30 °C in the dark. The cells were then washed twice and incubated in the dark in control medium (or pretreated with drugs) for 0.5–5 h before the start of the experiment. Fura 2 fluorescence (at 510 nm) was measured in cell populations with a PerkinElmer LS50B fluorescence spectrophotometer. Background fluorescence was determined at the end of the experiment by quenching the fura 2 fluorescence for 10–15 min in the presence of 1 μM ionomycin and 6 mM MnCl2 in Ca2+-free medium. After background fluorescence was subtracted, the ratio of fluorescence at 340 nm excitation to that at 380 nm was calculated and calibrated in terms of [Ca2+]i as described previously [8].
Mn2+-entry measurements
The rates at which Mn2+ enters the cells to quench fura 2 fluorescence were measured in cell populations after excitation at 358 nm, the wavelength that was empirically determined to be minimally sensitive to changes in [Ca2+]. Changes in fluorescence intensity were measured while 150 μM MnCl2 was present in Ca2+-free control medium (CaCl2 was omitted). Rates of fluorescence quench were determined using least-squares linear regression analysis (SigmaPlot 2000 software; SPSS, Chicago, IL, U.S.A.) of the change in emitted fluorescence in 1 min time periods (120 data points). Mn2+ entry was also measured in individual cells using a microscope-based digital image analysis system (Compix, Cranberry Township, PA, U.S.A.) that allowed for recording from approx. 10–15 cells within the microscope field. Sequential excitation at 340, 359 and 380 nm allowed simultaneous recording of Mn2+ entry (at 359 nm) and [Ca2+]i (as indicated by changes in the 340:380 fluorescence ratio). All three wavelengths were recorded at acquisition rates of approx. 1 s−1. Fluorescence intensity in single cells was measured using Compix SimplePCI software. For the analysis of Mn2+-entry rates, fluorescence intensity after excitation at 359 nm (F) was normalized to the mean fluorescence recorded for 60 s, just before the addition of MnCl2 (F0); regression analysis from 2 min periods of normalized fluorescence (F/F0) was used to estimate quench rates.
Materials
Amphotericin B was obtained from Calbiochem (La Jolla, CA, U.S.A.); AVP, [β-mercapto-β,β-cyclopentamethylenepropionyl1, O-Et-Tyr2, Val4, Arg8]-vasopressin, CPA, ionomycin, nimodipine, spermine chloride and thapsigargin were obtained from Sigma-Aldrich (St. Louis, MO, U.S.A.); fura 2/AM and Pluronic F127 were from Molecular Probes (Eugene, OR, U.S.A.).
LOE 908 was generously provided by Boehringer Ingelheim Pharmaceuticals (Ridgefield, CT, U.S.A.). Solubilization of LOE 908 was accomplished by preparing a 10 mM stock solution in deionized water and then adding the appropriate volume of this stock to a volume of control medium before adjusting the pH to its final value.
RESULTS
We used electrophysiological techniques to measure ISOC activated by depletion of Ca2+ stores. As shown in Figure 1(A), ISOC is activated incrementally by the addition of 1 and 100 nM AVP and it stabilizes after the addition of 10 μM CPA, a reversible inhibitor of the sarco/endoplasmic reticulum Ca2+-ATPase. ISOC activity reverses rapidly on washout of CPA (Figure 1A). The same inwardly rectifying current developed as a result of passive emptying of Ca2+ stores with 1 μM thapsigargin (irreversible inhibitor of sarcoplasmic reticulum Ca2+-ATPase; Figures 1C and 1D) or by cell dialysis with 10 mM BAPTA (<1 nM free Ca2+ in the pipette) in the ruptured patch configuration (Figures 1E and 1F).
ISOC activated by emptying of Ca2+ stores with AVP, CPA, thapsigargin or BAPTA have similar current densities measured at −115 mV (−0.60±0.10 pA/pF AVP-activated ISOC, n=6; −0.49±0.10 pA/pF thapsigargin-activated ISOC, n=8; −0.55±0.03 pA/pF BAPTA-activated ISOC, n=26; −0.39±0.05 pA/pF CPA-activated ISOC, n=5), have the same-shaped I–V curves, reversed at the same voltage and were each blocked by Gd3+.
Replacement of 100 mM NMDG+ in the external solution with 100 mM Na+ did not alter the reverse potential or current density (Figure 1G), suggesting that ISOC is relatively selective for Ca2+ compared with Na+. In agreement with fura 2 fluorescence measurements of CCE, ISOC is insensitive to 30 μM LOE 908 (Figure 1H), a channel-blocking drug that was reported to inhibit NCCE without affecting CCE [3]. On the basis of its activation by treatments that actively or passively deplete intracellular Ca2+ stores, its ability to conduct Ca2+, its sensitivity to μM Gd3+ and insensitivity to LOE 908, we hypothesize that ISOC corresponds to the CCE pathway identified by fura 2 fluorescence studies.
On the basis of this hypothesis, the reciprocal regulation model of Moneer et al. [3,6] would predict that ISOC would be active only after AVP removal and that AVP would inhibit thapsigargin-activated ISOC. Using perforated patch whole-cell voltage clamp, we find that ISOC is active in the presence of 100 nM AVP, with no apparent rebound on the removal of AVP (Figure 2A). We also found that thapsigargin-activated ISOC is not inhibited by 100 nM AVP (Figure 2B).
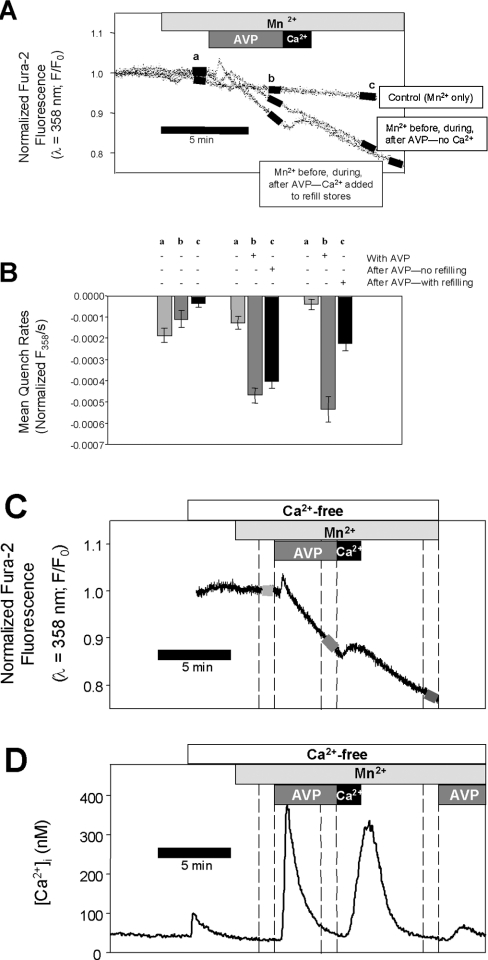
To examine the apparent disagreement between the electrophysiology results and the reciprocal regulation model proposed by Moneer and Taylor [3], we used fura 2 fluorescence techniques, specifically focusing on the proposed inhibition of CCE by AVP. Previous studies [1] suggested that the CCE pathway is permeable to Mn2+, whereas the NCCE pathway does not conduct Mn2+. The reciprocal regulation theory [3] would predict that, during AVP exposure, there would be little enhancement of Mn2+ entry, but a robust increase in Mn2+ entry after the removal of AVP. We found that Mn2+ entry was significantly increased in the presence of 100 nM AVP (by 4.5±1.2-fold, n=4, P<0.01, paired t test) and there was no rebound of Mn2+ entry after AVP removal (Figure 3). The AVP-stimulated Mn2+ entry may be attributed to CCE since (i) it did not require continued receptor activation (the stimulated rate was maintained after the removal of AVP in the Ca2+-free medium) and (ii) brief exposure to extracellular Ca2+ (to refill partially the Ca2+ stores) significantly decreased the rate of Mn2+ entry (by 58±4%, n=3, P<0.05; Figures 3B and 3C).
Figure 3. AVP stimulates Mn2+ entry: no rebound after AVP removal.
(A) Unidirectional Mn2+ entry was measured at three time points (labelled a, b and c) in fura 2-loaded A7r5 cell monolayers based on the rate of quench of fura 2 fluorescence (λex=358 nm). Three sets of experiments were performed, represented by the three labelled traces in the top panel: the time course of Mn2+ entry in Ca2+-free medium without any treatment (Control); the time course of Mn2+ entry before, during and after treatment with 100 nM AVP; the time course of Mn2+ entry before and during 100 nM AVP and after a brief introduction of 10 mM CaCl2 to allow partial refilling of the intracellular Ca2+ stores. (B) The rates of fluorescence quench were measured (linear regression) at each of the three time points (black bars superimposed on traces on A) for each set of experiments (n=3–4) and are summarized in the bar graph. The results indicate that capacitative Mn2+ entry is enhanced while AVP is present and is not further increased after AVP removal. (D) Changes in [Ca2+]i that occur during the treatments for the third set of Mn2+ quench experiments (AVP followed by Ca2+). [Ca2+]i was estimated from the 340:380 fura 2 fluorescence ratios recorded from a different coverslip treated with the same solutions. In this case, the cells were exposed a second time to AVP to demonstrate that transient exposure to extracellular Ca2+ enabled the subsequent additional release of intracellular Ca2+. The representative Mn2+-entry recording is aligned temporally on (C) above the [Ca2+]i recording, demonstrating that the rates of quench were determined at those time points when cytosolic [Ca2+] was low and would not interfere with the Mn2+-entry measurements.
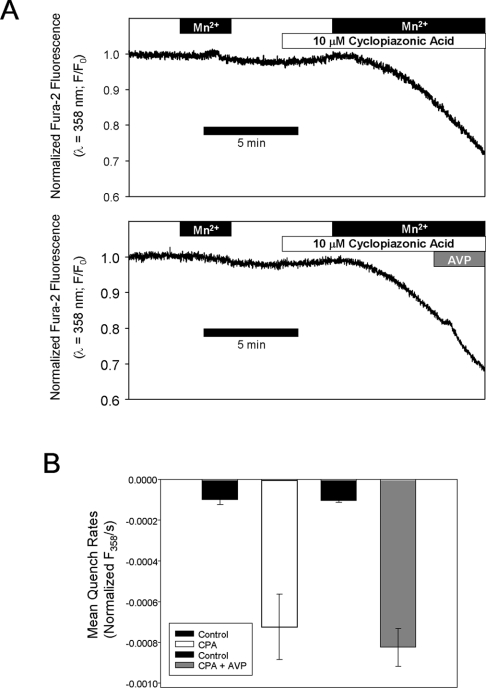
The question of whether AVP inhibits Mn2+ entry after store depletion was also re-examined. Mn2+ entry was measured before or during exposure to CPA; CPA stimulated Mn2+ entry by 7.4±0.8-fold (n=3). No change in Mn2+ entry was observed when AVP (100 nM) was added after the stabilization of CPA-activated Mn2+ entry (8.0±0.8-fold increase over basal, n=3, P>0.5 compared with CPA alone; Figure 4). These results are consistent with the conclusion of Byron and Taylor [1] that AVP does not inhibit capacitative Mn2+ entry, but cannot be readily reconciled with the results of Moneer and Taylor [3].
Figure 4. AVP does not inhibit CPA-activated Mn2+ entry.
(A) Quench of fura 2 fluorescence due to unidirectional Mn2+ entry was measured in A7r5 cell populations before or during exposure to 10 μM CPA. CPA robustly increases Mn2+ entry and the rates of entry were not different when 100 nM AVP was added after maximal activation of Mn2+ entry by CPA. (B) Quench rates were estimated by least-squares regression analysis of the fluorescence recording during the final 60 s of each Mn2+ exposure; results represent the means for three independent experiments (error bars indicate S.E.M.).
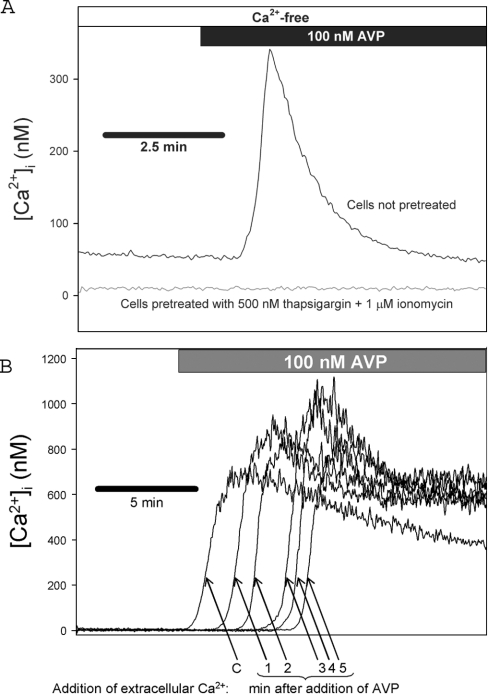
A7r5 cells were not derived by cloning [12] and therefore might be expected to display heterogeneity among cells in a population. We used single-cell imaging techniques to examine the heterogeneity of single-cell responses using a pretreatment method established by Byron and Taylor [1] and also used by Broad et al. [4] and Moneer and Taylor [3]. A7r5 cells are pretreated with a combination of ionomycin (1 μM) and thapsigargin (500 nM) to deplete fully the intracellular Ca2+ stores. The effectiveness of this treatment is demonstrated in Figure 7(A) where a robust Ca2+ release response to 100 nM AVP in untreated cells is compared with an undetectable Ca2+ release in the ionomycin/thapsigargin-pretreated cells. Figure 5(A) shows representative recordings of Mn2+ entry in individual A7r5 cells in the absence (upper left panels) or presence of 100 nM AVP (upper right panels). Capacitative Mn2+ entry produced a steady decrease in fura 2 fluorescence measured at the Ca2+-insensitive wavelength (359 nm; Figure 5A, upper panels), which was not altered by the presence of 100 nM AVP. The Ca2+-sensitive fluorescence ratio measured in the same cells (340:380; Figure 5A, lower panels) confirms that AVP did not detectably release additional Ca2+ from intracellular stores. Analysis of 102 cells from four independent experiments indicates that there was no effect of AVP on mean Mn2+-entry rates (Figure 5B). We also examined the distribution of rates among individual cells comparing untreated and AVP-treated groups (Figure 5C). There was no significant difference in the distribution of rates, although there was a tendency towards a time-dependent decrease in quench rates in the controls, which was decreased by AVP, contrary to the predictions of the reciprocal regulation model.
Figure 7. CCE is enhanced, not inhibited, by AVP.
A7r5 cells were pretreated with 500 nM thapsigargin +1 μM ionomycin in Ca2+-free medium to deplete intracellular Ca2+ stores. (A) AVP-stimulated release of intracellular Ca2+ stores was estimated from fura 2 fluorescence (340:380 ratios) recorded from A7r5 cell populations during exposure to 100 nM AVP in Ca2+-free medium in untreated controls (upper trace) or cells pretreated with 500 nM thapsigargin +1 μM ionomycin (lower trace). These results demonstrate the effectiveness of the pretreatment for depletion of Ca2+ stores. (B) [Ca2+]i was estimated from fura 2 fluorescence ratios (340:380) recorded during the reintroduction of extracellular Ca2+ (1.5 mM) without [arrow labelled ‘C’ or at various times (indicated below arrows) after the addition of 100 nM AVP]. Representative traces from individual coverslips are superimposed. Results are representative of three independent experiments.
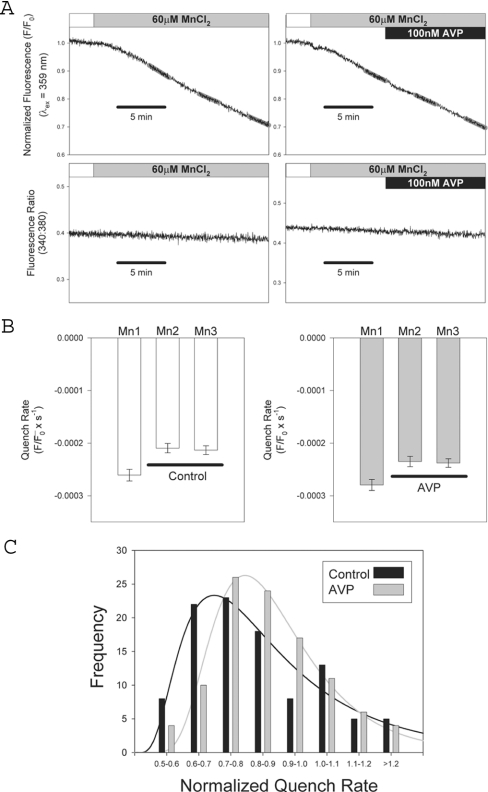
Figure 5. Analysis of store-operated Mn2+ entry in single cells: no effect of AVP.
(A) Representative recordings of fura 2 fluorescence from single A7r5 cells pretreated with 500 nM thapsigargin +1 μM ionomycin; upper panels show the quench of fura 2 fluorescence due to unidirectional Mn2+ entry at 359 nm excitation; lower panels show 340:380 ratios recorded simultaneously from the same cells. Right panels show responses recorded from a cell treated with 100 nM AVP (black bar). Linear regression analysis was used to measure fluorescence quench rates at three time points (indicated by grey regression lines labelled Mn1, Mn2 and Mn3 superimposed on traces). (B) Summarized data from 102 cells (51 untreated controls and 51 treated with 100 nM AVP); quench rates measured at each of the three time points indicated above (mean±S.E.M.; no significant difference between control and AVP-treated). (C) Histogram analysis of the distribution of rates comparing control and AVP-treated cells. For each cell, the quench rates measured at time points Mn2 and Mn3 were normalized by dividing by the rates measured at Mn1. There was no significant difference in normalized quench rates between control and AVP-treated (Mann–Whitney Rank Sum Test, P>0.05).
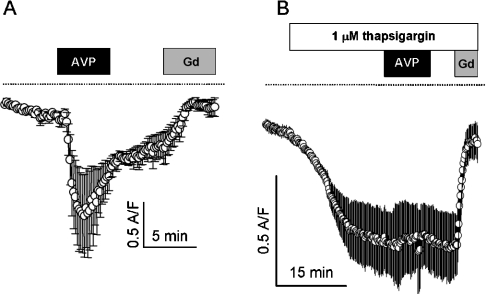
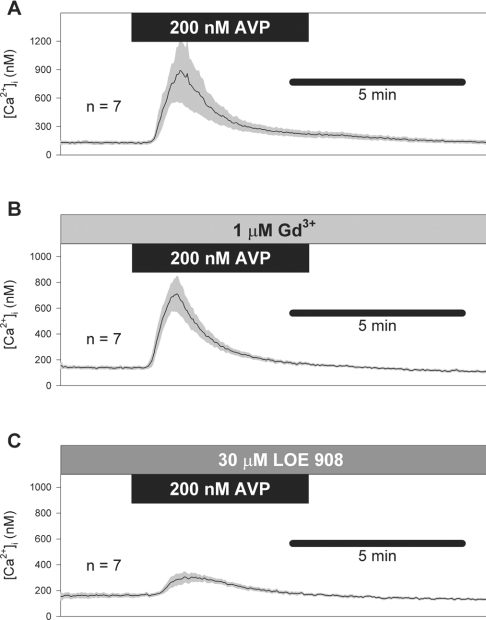
Results of Broad et al. [4] and Moneer and Taylor [3] indicate that 1 μM Gd3+ selectively blocks CCE and 30 μM LOE 908 selectively blocks NCCE. Moneer and Taylor [3] report a robust rebound of [Ca2+]i after the removal of 200 nM AVP in the presence of 30 μM LOE 908, which they attribute to CCE. Using the same treatment method [same durations of treatment and concentrations of drugs and AVP, same perfusion rate (17 ml/min), addition of 100 nM [β-mercapto-β,β-cyclopentamethylenepropionyl1, O-Et-Tyr2, Val4, Arg8]-vasopressin, a V1a vasopressin receptor antagonist, at the time of AVP washout [3], we found no rebound of [Ca2+]i after the removal of AVP (200 nM; Figure 6). Gd3+ (1 μM) had little effect on AVP-induced [Ca2+]i responses (Figure 6B). LOE 908 (30 μM) inhibited the [Ca2+]i response to AVP, but no rebound Ca2+ transient was observed at the time of AVP removal (Figure 6C).
Figure 6. No rebound of Ca2+ entry after AVP removal.
[Ca2+]i responses to 200 nM AVP were measured by the method of Moneer and Taylor [3]. Mean responses (solid traces)±S.E.M. (shaded error bars) are shown for AVP alone (A), AVP in the presence of Gd3+ (B) and AVP in the presence of LOE 908 (C). Note the absence of rebound transient on removal of AVP. As described by Moneer and Taylor [3], a V1a vasopressin receptor antagonist ([β-mercapto-β,β-cyclopentamethylenepropionyl1, O-Et-Tyr2, Val4, Arg8]-vasopressin; 100 nM) was added at the time of AVP washout.
Moneer et al. [6] reported that AVP treatment nearly abolishes the Ca2+ transient that results when extracellular Ca2+ is reintroduced after the depletion of intracellular Ca2+ stores. We performed a set of experiments in which intracellular Ca2+ stores were first depleted with 500 nM thapsigargin and 1 μM ionomycin in Ca2+-free medium (a similar method was used by Moneer et al. [6]). Fura 2 fluorescence ratios were then recorded during the reintroduction of extracellular Ca2+ (1.5 mM) without or at various times after the addition of 100 nM AVP (Figure 7). In three sets of experiments, the mean peak [Ca2+]i response was at least 30% larger, at all time points after AVP addition, compared with the control response measured in the absence of AVP. The enhanced peak is consistent with the activation of an additional Ca2+-entry pathway (NCCE) by AVP, but inconsistent with the reciprocal regulation model of Moneer et al. [3,6].
DISCUSSION
Our present findings confirm earlier results from Byron and Taylor [1] demonstrating that AVP activates capacitative Ca2+ or Mn2+ entry, but neither enhances nor inhibits this cation-entry pathway once it has been maximally activated by passive store depletion. We also identify, for the first time in A7r5 cells, the electrophysiological equivalent of CCE, a store-operated cation current, which is activated by AVP or other treatments that result in the depletion of intracellular Ca2+ stores. This current (ISOC) behaves in accordance with our fluorescence measurements of bivalent cation entry.
In several previous studies [13–16], ISOC was not detected in A7r5 cells despite abundant evidence that CCE could be detected using fura 2 fluorescence. Iwamuro et al. [13], Iwasawa et al. [14], Jung et al. [15] and Nakajima et al. [16] failed to detect ISOC at steady −40 to −60 mV holding potential in external solutions containing 0.2–1.8 mM Ca2+. We were also unable to detect ISOC under these conditions (results not shown). However, methods similar to those that we have used in the present study have been used by other investigators to detect store-operated currents in vascular smooth-muscle cells [17].
In A7r5 cells, ISOC is a relatively Ca2+-selective, inwardly rectifying, Gd3+-sensitive cation current (Figures 1 and 2), which is distinct from at least two non-selective cation currents that may be detected in these cells (L. I. Brueggemann, L. L. Cribbs and K. L. Byron, unpublished work). Its identification as a store-operated current is based on: (i) its activation by passive store depletion independent of ligand–receptor interactions, e.g. by inhibition of sarco/endoplasmic reticulum Ca2+-ATPase with thapsigargin or CPA or by dialysis with 10 mM BAPTA in a ruptured patch configuration; (ii) its pharmacological properties (Gd3+ sensitivity, LOE 908-insensitivity); and (iii) its ability to conduct Ca2+. The pharmacological properties of ISOC are consistent with fura 2 fluorescence measurements of CCE, which demonstrated that CCE was distinguished from NCCE by its sensitivity to low [Gd3+] [4] and its insensitivity to LOE 908 [3]. Its electrophysiological characteristics resemble those of ISOC measured in vascular smooth-muscle cells by other investigators [17,18]. Furthermore, we found that ISOC is significantly inhibited by treatment with an antisense construct to TRPC1 [19], a member of the transient receptor potential family of non-selective cation channels, which has been implicated by other investigators as a store-operated Ca2+ channel in vascular smooth muscles [20,21].
Activation of ISOC by 100 nM AVP reproducibly reached a peak and then partially inactivated (Figures 1A and 2A). No inactivation of ISOC was apparent after it was activated by passive store depletion with thapsigargin, CPA or BAPTA dialysis; nor when AVP was applied after full activation of the current by thapsigargin (Figure 2B). A possible explanation for this observation is that partial refilling of the Ca2+ stores occurs during AVP treatment (this would tend to shut off the store-operated pathway and would not occur with the passive store depletion regimens or when AVP is added during thapsigargin treatment). Receptor desensitization might account for such an observation, but feedback inhibition on entry channels through a signalling pathway is an unlikely explanation because it would be expected to produce inactivation when AVP is added during thapsigargin treatment.
A reciprocal regulation model proposed by Moneer and Taylor [3] predicts that AVP should inhibit ISOC. The activation of ISOC by AVP and the absence of a rebound activation on AVP removal, combined with the observation that AVP did not inhibit thapsigargin-activated ISOC, led us to re-examine the reciprocal regulation model, applying the fura 2 fluorescence methods used in the studies on which the model was based [3]. Our present findings are consistent with earlier studies of AVP-stimulated Ca2+ entry in A7r5 cells [1,4,5], but cannot be readily reconciled with the results of Moneer and Taylor [3] and Moneer et al. [6], which suggest that AVP inhibits store-operated Ca2+ or Mn2+ entry. We have not been able to reproduce some of the findings of Moneer et al. [3,6] despite our attempts to mimic as closely as possible the conditions described in their studies. For example, we did not observe any detectable rebound of [Ca2+]i on AVP removal in the presence of LOE 908 (Figure 6C), whereas Figure 7(A) in [3] shows a huge rebound transient in which [Ca2+]i reaches a peak at approx. 800 nM in amplitude. We observed an enhancement by AVP of the Ca2+ transient when extracellular Ca2+ was reintroduced to cells with depleted Ca2+ stores (Figure 7), whereas Figures 3(A) and 3(B) in [6] show that AVP completely abolishes this transient.
These contradictory findings suggest that something was different in the studies of Moneer et al. [3,6], but where that difference lies cannot be readily discerned from their description of the experimental design and methods. All of the methods for cell culture and fura 2 fluorescence appear to be essentially the same as those originally described by Byron and Taylor [7]. There were some subtle differences in the experimental design for measuring Mn2+ entry: in the experiments described by Moneer and Taylor [3], AVP was present for only 1 min and fluorescence quench rates were determined by regression analysis from only the final 20 s of that treatment. Other experiments by Moneer and Taylor [3] used much longer AVP treatments and indicated that the effects of AVP on CCE persisted for at least 5 min, so it is not clear why such a brief exposure was used for their Mn2+-entry measurements. We have found that analysis of longer recordings (1–2 min) are necessary to obtain a linear regression with a correlation coefficient greater than 0.5 (in the present study, we excluded results with fits that did not meet this criterion) and that longer treatments are necessary to obtain stable quench rates (Figure 4). There is no evidence from the three earlier studies [1,4,5] or the present study that AVP inhibits CCE. In contrast, the results of Moneer et al. indicate that AVP does inhibit CCE. It is possible that the cells that were used by Moneer et al. represent a subpopulation of A7r5 cells with different properties, but the results from our analysis of single-cell responses do not identify any cell with the expected properties within our A7r5 cell population. If the reciprocal regulation phenotype has such a limited expression, the broad applicability of this model postulated by Taylor [22,23] may be questionable. The general stability of the A7r5 cell line does not appear to be in question because the cells used in the present study were derived from the same cells used throughout the 1990s by the Taylor laboratory [1,4,5,7] and the present results agree with the results of those earlier studies.
Reciprocal or co-ordinated regulation of multiple Ca2+-entry pathways has been reported in several cell types and involving several agonist-stimulated signalling pathways (see discussion by Moneer and Taylor [3]). Most convincingly, activation of an NCCE pathway (ARC channels) by muscarinic agonists may be inhibited by sustained Ca2+ signals derived from store-operated Ca2+ entry at high agonist concentrations [24,25]. Our present findings do not rule out the possibility of such a co-ordinate regulation or the possibility that different Ca2+-entry pathways may be activated by different concentrations of agonists, as suggested by early studies of AVP effects in A7r5 cells [1,2,4]. However, our findings do call into question the notion that CCE is inhibited by AVP in vascular smooth-muscle cells.
Regulation of Ca2+ entry in vascular smooth-muscle cells is of crucial importance to cardiovascular physiology since it regulates the contractile state of these cells and hence the diameter of blood vessels as a primary determinant of blood flow and blood pressure. Understanding how vasoconstrictor hormones regulate Ca2+ entry may provide a better understanding of cardiovascular diseases and improved therapeutic tools for the treatment of these diseases. Our findings bring to light a controversy in the literature of this field. Confirmation by independent laboratories may be required before additional weight is given to support or refute the reciprocal regulation model. In addition, new electrophysiological evidence such as that provided in the present study suggests a means to help resolve the controversy and understand the regulation of Ca2+ entry by vasopressin.
Acknowledgments
This work was supported by the John and Marian Falk Trust for Medical Research and the National Heart, Lung and Blood Institute (R01HL070670; to K. L. B.).
References
- 1.Byron K. L., Taylor C. W. Vasopressin stimulation of Ca2+ mobilization, two bivalent cation entry pathways and Ca2+ efflux in A7r5 rat smooth muscle cells. J. Physiol. (Cambridge, U.K.) 1995;485:455–468. doi: 10.1113/jphysiol.1995.sp020742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Byron K. L. Vasopressin stimulates Ca2+ spiking activity in A7r5 vascular smooth muscle cells via activation of phospholipase A2. Circ. Res. 1996;78:813–820. doi: 10.1161/01.res.78.5.813. [DOI] [PubMed] [Google Scholar]
- 3.Moneer Z., Taylor C. W. Reciprocal regulation of capacitative and non-capacitative Ca2+ entry in A7r5 vascular smooth muscle cells: only the latter operates during receptor activation. Biochem. J. 2002;362:13–21. doi: 10.1042/0264-6021:3620013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Broad L. M., Cannon T. R., Taylor W. A non-capacitative pathway activated by arachidonic acid is the major Ca2+ entry mechanism in rat A7r5 smooth muscle cells stimulated with low concentrations of vasopressin. J. Physiol. (Cambridge, U.K.) 1999a;517:121–134. doi: 10.1111/j.1469-7793.1999.0121z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broad L. M., Cannon T. R., Short A. D., Taylor C. W. Receptors linked to polyphosphoinositide hydrolysis stimulate Ca2+ extrusion by a phospholipase C-independent mechanism. Biochem. J. 1999b;342:199–206. [PMC free article] [PubMed] [Google Scholar]
- 6.Moneer Z., Dyer J. L., Taylor C. W. Nitric oxide co-ordinates the activities of the capacitative and non-capacitative Ca2+-entry pathways regulated by vasopressin. Biochem. J. 2003;370:439–448. doi: 10.1042/BJ20021104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byron K. L., Taylor C. W. Spontaneous Ca2+ spiking in a vascular smooth muscle cell line is independent of the release of intracellular Ca2+ stores. J. Biol. Chem. 1993;268:6945–6952. [PubMed] [Google Scholar]
- 8.Fan J., Byron K. L. Ca2+ signalling in rat vascular smooth muscle cells: a role for protein kinase C at physiological vasoconstrictor concentrations of vasopressin. J. Physiol. (Cambridge, U.K.) 2000;524:821–831. doi: 10.1111/j.1469-7793.2000.00821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wayman C. P., McFadzean I., Gibson A., Tucker J. F. Two distinct membrane currents activated by cyclopiazonic acid-induced calcium store depletion in single smooth muscle cells of the mouse anococcygeus. Br. J. Pharmacol. 1996;117:566–572. doi: 10.1111/j.1476-5381.1996.tb15228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartzell H. C., Zhiqiang Q. Chloride currents in acutely isolated Xenopus retinal pigment epithelial cells. J. Physiol. (Cambridge, U.K.) 2003;549:453–469. doi: 10.1113/jphysiol.2003.040428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nilius B. Calcium-impermeable monovalent cation channels: a TRP connection? Br. J. Pharmacol. 2003;138:5–7. doi: 10.1038/sj.bjp.0705073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kimes B. W., Brandt B. L. Characterization of two putative smooth muscle cell lines from rat thoracic aorta. Exp. Cell Res. 1976;98:349–366. doi: 10.1016/0014-4827(76)90446-8. [DOI] [PubMed] [Google Scholar]
- 13.Iwamuro Y., Miwa S., Zhang X. F., Minowa T., Enoki T., Okamoto Y., Hasegawa H., Furutani H., Okazawa M., Ishikawa M., et al. Activation of three types of voltage-independent Ca2+ channel in A7r5 cells by endothelin-1 as revealed by a novel Ca2+ channel blocker LOE 908. Br. J. Pharmacol. 1999;126:1107–1114. doi: 10.1038/sj.bjp.0702416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwasawa K., Nakajima T., Hazama H., Goto A., Shin W. S., Toyo-oka T., Omata M. Effects of extracellular pH on receptor-mediated Ca2+ influx in A7r5 rat smooth muscle cells: involvement of two different types of channel. J. Physiol. (Cambridge, U.K.) 1997;503:237–251. doi: 10.1111/j.1469-7793.1997.237bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jung S., Strotmann R., Schultz G., Plant T. TRPC6 is a candidate channel involved in receptor-stimulated cation currents in A7r5 smooth muscle cells. Am. J. Physiol. Cell Physiol. 2002;282:C347–C359. doi: 10.1152/ajpcell.00283.2001. [DOI] [PubMed] [Google Scholar]
- 16.Nakajima T., Hazama H., Hamada E., Wu S.-N., Igarashi K., Yamashita T., Seyama Y., Omata M., Kurachi Y. Endothelin-1 and vasopressin activate Ca2+-permeable non-selective cation channels in aortic smooth muscle cells: mechanism of receptor-mediated Ca2+ influx. J. Mol. Cell. Cardiol. 1996;28:707–722. doi: 10.1006/jmcc.1996.0066. [DOI] [PubMed] [Google Scholar]
- 17.Albert A. P., Large W. A. A Ca2+-permeable non-selective cation channel activated by depletion of internal Ca2+ stores in single rabbit portal vein myocytes. J. Physiol. (Cambridge, U.K.) 2002;538:717–728. doi: 10.1113/jphysiol.2001.013101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Curtis T. M., Scholfield C. N. Nifedipine blocks Ca2+ store refilling through a pathway not involving L-type Ca2+ channels in rabbit arteriolar smooth muscle. J. Physiol. (Cambridge, U.K.) 2001;532:609–623. doi: 10.1111/j.1469-7793.2001.0609e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Byron K. L., Brueggemann L. I., Cribbs L. L. TRPC1 antisense inhibits currents and Mn2+ entry activated by store-depletion in A7r5 vascular smooth muscle cells. FASEB. J. 2004;18:A305. [Google Scholar]
- 20.Golovina V. A., Platoshyn O., Bailey C. L., Wang J., Limsuwan A., Sweeney M., Rubin L. J., Yuan J. X. Upregulated TRP and enhanced capacitative Ca2+ entry in human pulmonary artery myocytes during proliferation. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H746–H755. doi: 10.1152/ajpheart.2001.280.2.H746. [DOI] [PubMed] [Google Scholar]
- 21.Sweeney M., Yu Y., Platoshyn O., Zhang S., McDaniel S. S., Yuan J. X.-J. Inhibition of endogenous TRP1 decreases capacitative Ca2+ entry and attenuates pulmonary artery smooth muscle cell proliferation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002;283:L144–L155. doi: 10.1152/ajplung.00412.2001. [DOI] [PubMed] [Google Scholar]
- 22.Taylor C. W. Controlling calcium entry. Cell (Cambridge, Mass.) 2002;111:767–769. doi: 10.1016/s0092-8674(02)01197-2. [DOI] [PubMed] [Google Scholar]
- 23.Taylor C. W. Regulation of Ca2+ entry pathways by both limbs of the phosphoinositide pathway. Novartis Found. Sym. 2002;246:91–101. [PubMed] [Google Scholar]
- 24.Mignen O., Thompson J. L., Shuttleworth T. J. Reciprocal regulation of capacitative and arachidonate-regulated noncapacitative Ca2+ entry pathways. J. Biol. Chem. 2001;276:35676–35683. doi: 10.1074/jbc.M105626200. [DOI] [PubMed] [Google Scholar]
- 25.Mignen O., Thompson J. L., Shuttleworth T. J. Calcineurin directs the reciprocal regulation of calcium entry pathways in nonexcitable cells. J. Biol. Chem. 2003;278:40088–40096. doi: 10.1074/jbc.M306365200. [DOI] [PubMed] [Google Scholar]