Abstract
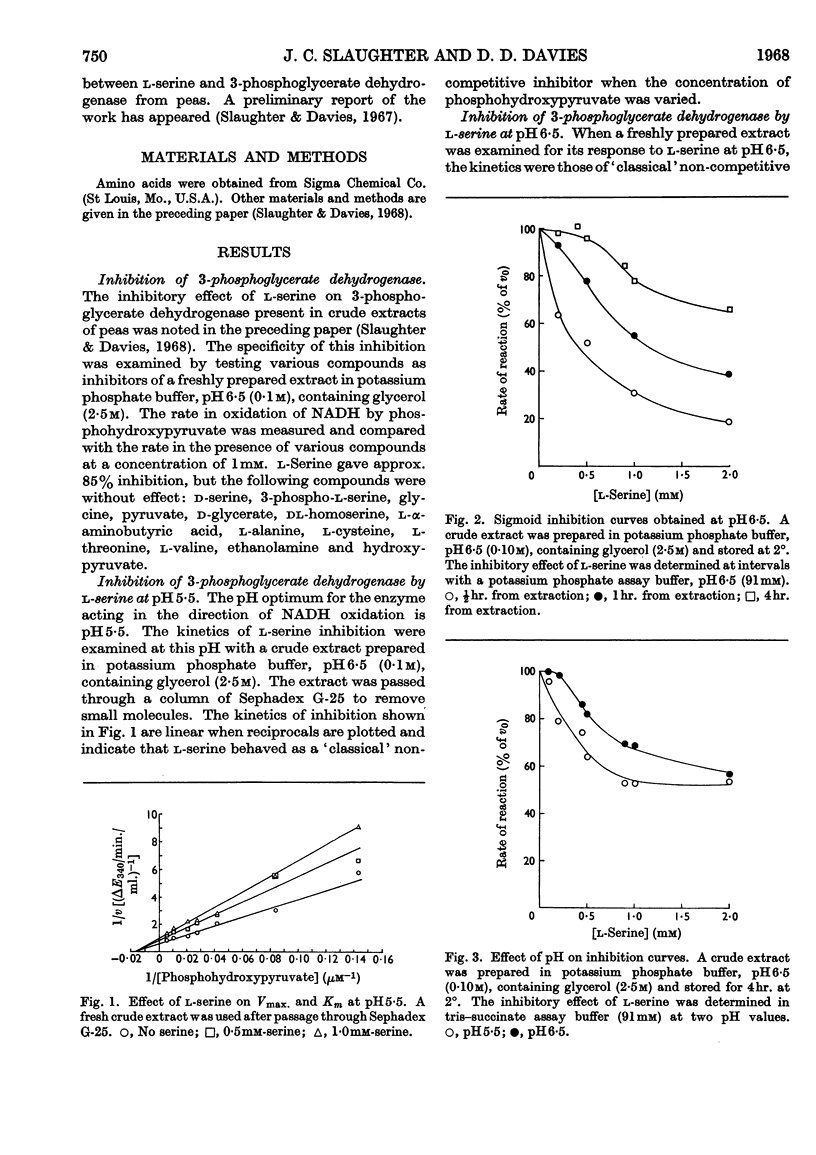
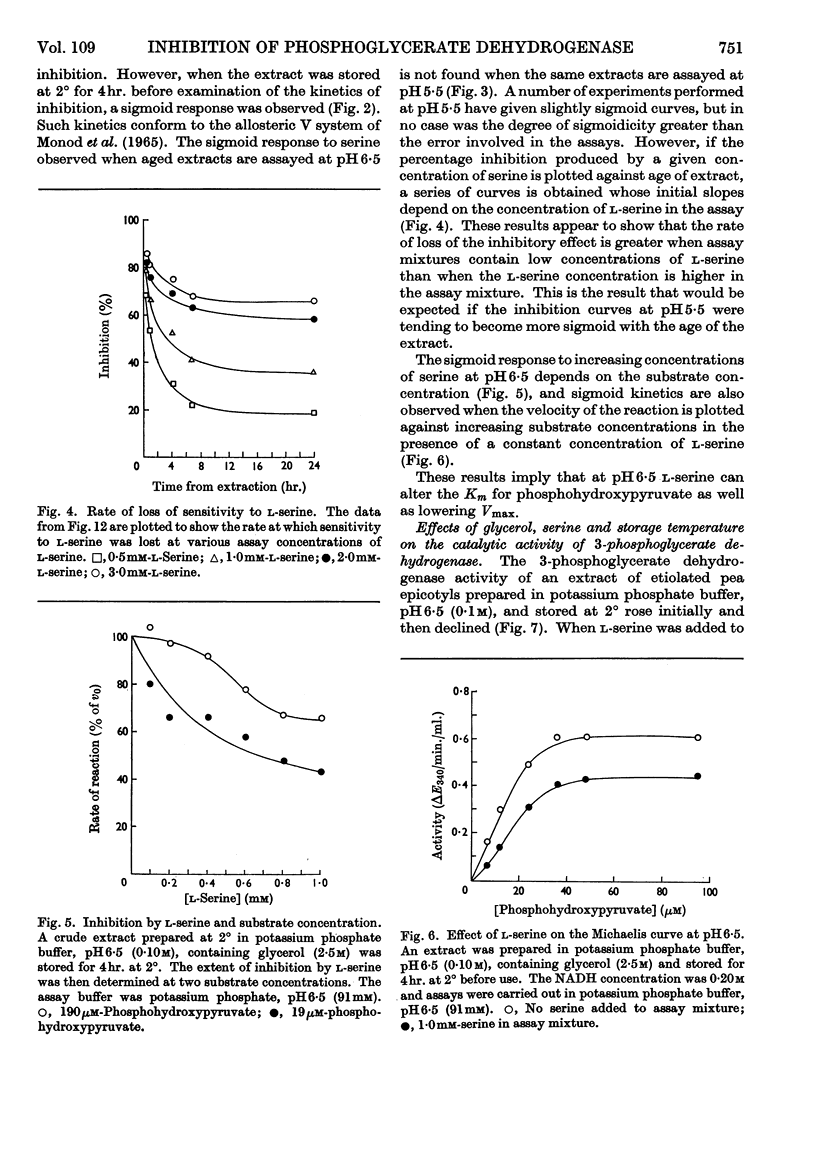
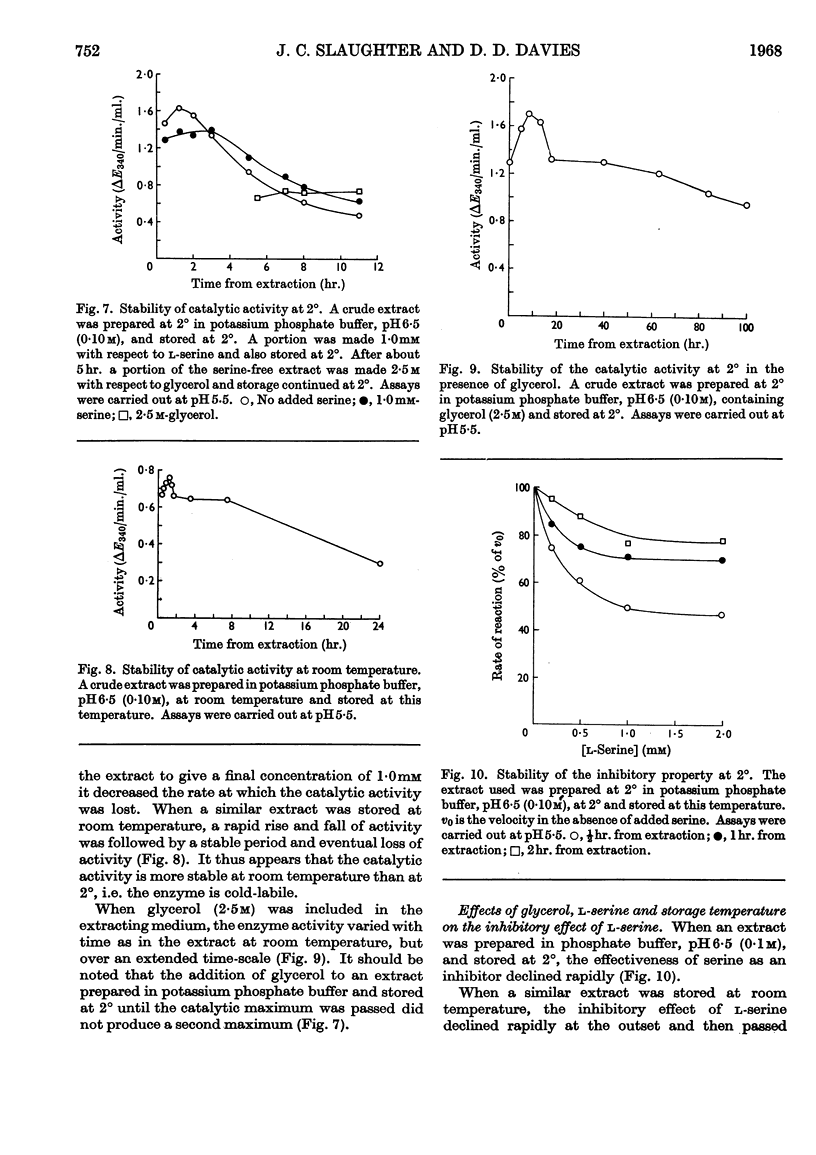
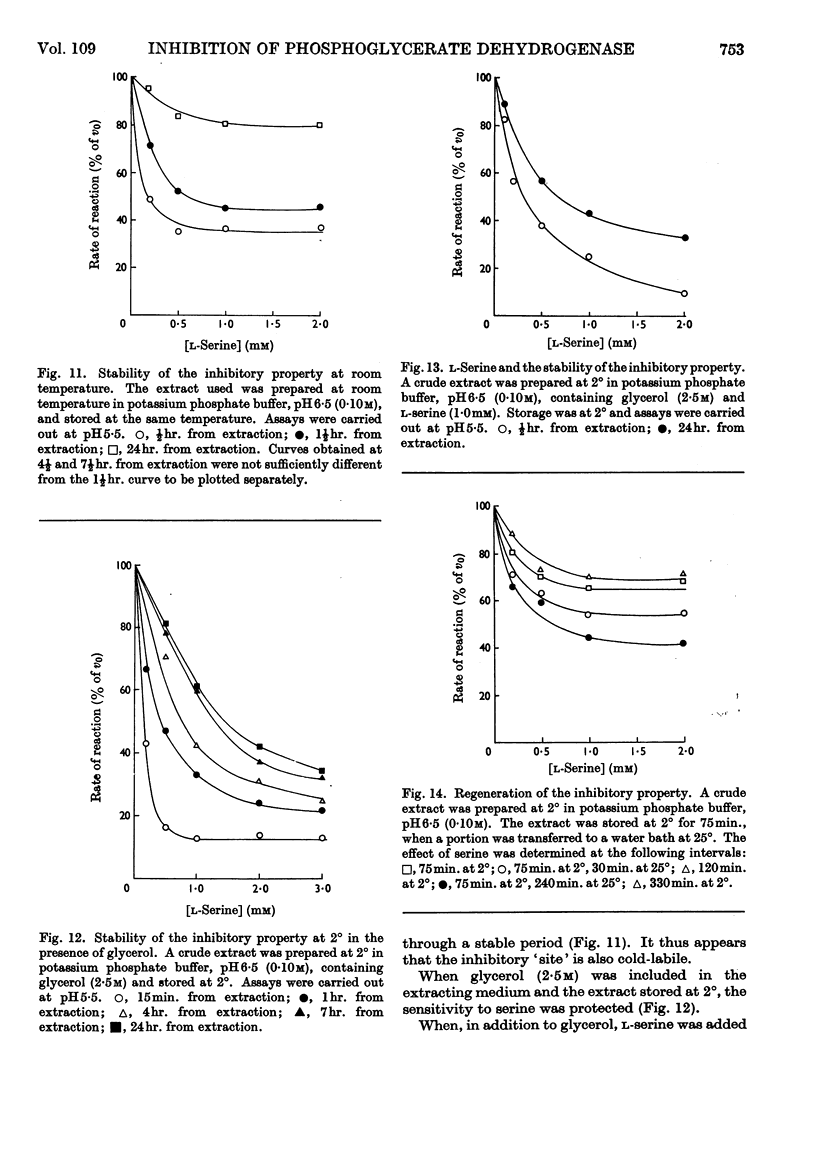
1. l-Serine was shown to be a highly specific inhibitor of 3-phosphoglycerate dehydrogenase. 2. 3-Phosphoglycerate dehydrogenase is cold-labile with respect to its catalytic activity and to sensitivity to serine. 3. l-Serine protects the catalytic site as well as the inhibitor site. 4. Glycerol protects the catalytic site as well as the inhibitor site. 5. Serine acts as a `classical' non-competitive inhibitor of fresh preparations of 3-phosphoglycerate dehydrogenase. 6. `Aged' preparations when assayed at pH6·5 show sigmoid inhibition curves at saturating substrate concentrations. 7. A generalized model is advanced to account for the variation of the catalytic activity and the inhibitory effect of l-serine with time and conditions. 8. The possibility that the sigmoid kinetics of inhibition observed are an artifact of isolation is discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATKINSON D. E., HATHAWAY J. A., SMITH E. C. KINETICS OF REGULATORY ENZYMES. KINETIC ORDER OF THE YEAST DIPHOSPHOPYRIDINE NUCLEOTIDE ISOCITRATE DEHYDROGENASE REACTION AND A MODEL FOR THE REACTION. J Biol Chem. 1965 Jun;240:2682–2690. [PubMed] [Google Scholar]
- BORKENHAGEN L. F., KENNEDY E. P. The enzymatic exchange of L-serine with O-phospho-L-serine catalyzed by a specific phosphatase. J Biol Chem. 1959 Apr;234(4):849–853. [PubMed] [Google Scholar]
- Bridgers W. F. The biosynthesis of serine in mouse brain extracts. J Biol Chem. 1965 Dec;240(12):4591–4597. [PubMed] [Google Scholar]
- Koshland D. E., Jr, Némethy G., Filmer D. Comparison of experimental binding data and theoretical models in proteins containing subunits. Biochemistry. 1966 Jan;5(1):365–385. doi: 10.1021/bi00865a047. [DOI] [PubMed] [Google Scholar]
- Nakazawa A., Hayaishi O. On the mechanism of activation of L-threonine deaminase from Clostridium tetanomorphum by adenosine diphosphate. J Biol Chem. 1967 Mar 25;242(6):1146–1154. [PubMed] [Google Scholar]
- PIZER L. I. ENZYMOLOGY AND REGULATION OF SERINE BIOSYNTHESIS IN CULTURED HUMAN CELLS. J Biol Chem. 1964 Dec;239:4219–4226. [PubMed] [Google Scholar]
- PIZER L. I. THE PATHWAY AND CONTROL OF SERINE BIOSYNTHESIS IN ESCHERICHIA COLI. J Biol Chem. 1963 Dec;238:3934–3944. [PubMed] [Google Scholar]
- SANWAL B. D., STACHOW C. S., COOK R. A. A KINETIC MODEL FOR THE MECHANISM OF ALLOSTERIC ACTIVATION OF NICOTINAMIDE-ADENINE DINUCLEOTIDE-SPECIFIC ISOCRITIC DEHYDROGENASE. Biochemistry. 1965 Mar;4:410–421. doi: 10.1021/bi00879a006. [DOI] [PubMed] [Google Scholar]
- Slaughter J. C., Davies D. D. The allosteric nature of 3-phosphoglycerate dehydrogenase. Biochem J. 1967 Sep;104(3):49P–50P. [PMC free article] [PubMed] [Google Scholar]
- Slaughter J. C., Davies D. D. The isolation and characterization of 3-phosphoglycerate dehydrogenase from peas. Biochem J. 1968 Oct;109(5):743–748. doi: 10.1042/bj1090743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh D. A., Sallach H. J. Purification and properties of chicken liver D-3-phosphoglycerate dehydrogenase. Biochemistry. 1965 Jun;4(6):1076–1085. doi: 10.1021/bi00882a015. [DOI] [PubMed] [Google Scholar]


