Abstract
Aims: To investigate the deposition of complement components, C3d and C5b–9, and the expression of complement regulating factors (S protein, membrane cofactor protein (MCP; CD46), protectin (CD59), decay accelerating factor (DAF; CD55), and type 1 complement receptor (CR1; CD35)) in gastric cancers.
Methods: Specimens of gastric cancer were examined by immunohistochemistry and immunoelectron microscopy.
Results: Four complement regulating factors (S protein, MCP, protectin, and DAF) were expressed on gastric cancer cells, in ultrastructurally localised areas on the cell membrane. CR1 was not expressed. The staining intensity of DAF in both differentiated and undifferentiated adenocarcinomas was significantly higher than in histologically normal gastric epithelium. Furthermore, the staining intensity of DAF in gastric cancers showing a diffusely infiltrating growth pattern was higher than in gastric cancers showing an expanding growth pattern.
Conclusions: These data indicate that DAF may play a role in cancer cell infiltration and resistance in tumour cells.
Keywords: gastric cancer, complement regulatory protein, decay accelerating factor (CD55)
The complement system has been characterised extensively, both biochemically and functionally. It has been found to consist of more than 25 proteins acting dynamically together. The fact that more than half of these proteins function as regulators indicates that this highly potent biological effector system is kept under strict control.1 When complement components are deposited on host cells, complement regulating factors protect autologous cells from complement mediated cytotoxicity.2 The key enzymes of the complement cascade, C3/C5 convertases, are inhibited by the C3b receptor, type 1 complement receptor (CR1; CD35),3 decay accelerating factor (DAF; CD55),4 and membrane cofactor protein (MCP; CD46),5,6 by dissociation of the convertase complexes (CR1 and DAF) or by promotion of proteolytic inactivation of their non-catalytic subunits, C4b or C3b (CR1 and MCP). Two inhibitors of the membrane attack complex (C5b–9) have been described1,6,7: one has been termed S protein (vitronectin), and the other protectin (CD59). Previous studies have suggested that complement activation may play a role in tumour cytotoxicity,8–11 and a few have demonstrated the presence of complement regulatory proteins in malignant tumours.12–16 An excellent recent study has revealed significant complement mediated killing of microtumours treated with an antitumour antibody and a specific monoclonal antibody against the complement lysis inhibitor protein (CD59).17 However, there are no available data concerning the relation between the localisation of complement regulatory proteins on gastric cancer cells and clinicopathological features of their tumours.
“Previous studies have suggested that complement activation may play a role in tumour cytotoxicity, and a few have demonstrated the presence of complement regulatory proteins in malignant tumours”
Therefore, our present study aimed to investigate the expression of complement regulatory proteins in gastric cancers by immunohistochemistry and immunoelectron microscopy, and to assess the relation between the presence of these proteins on gastric cancer cells and the clinicopathological factors.
MATERIALS AND METHODS
Tissue samples
Resected gastric tissues were obtained at surgery from 28 patients, including one double cancer and one triple cancer. Eighteen lesions were differentiated adenocarcinomas, and 13 undifferentiated adenocarcinomas (table 1 ▶). In addition, 17 specimens of histologically normal (uninvolved) gastric tissue from the edges of the resected tissues were obtained from these patients. Neonatal gastric mucosa obtained from two postmortem cases was used as control. The tissue samples were sliced into sections approximately 5 mm thick, which were fixed in buffered 10% formalin, embedded in paraffin wax, and cut into 4 μm thick sections. The thin sections were stained with haematoxylin and eosin. A histological diagnosis including the infiltrative growth pattern and conclusive stage grouping was made according to the general rules for the gastric cancer study.18 Patterns of infiltrating growth of the tumours into the surrounding tissue are classified into the following three categories: INFα, the tumour shows expanding growth and a distinct border from the surrounding tissue; INFβ, this category is between INFα and INFγ; and INFγ, the tumour shows infiltrating growth and an indistinct border from the surrounding tissue.
Table 1.
Summary of pathological findings
| Histology | |||
| Neonatal gastric mucosa | n=2 | ||
| Uninvolved gastric mucosa | n=17 | ||
| Cancerous mucosa | n=31 (double cancer n=1, triple cancer n=1) | ||
| Differentiated adenocarcinoma | n=18 | Undifferentiated adenocarcinoma | n=13 |
| Papillary adenocarcinoma | n=5 | Poorly differentiated adenocarcinoma | n=12 |
| Tubular adenocarcinoma | n=13 | Signet ring cell carcinoma | n=1 |
| Clinicopathological findings | Differentiated adenocarcinoma | Undifferentiated adenocarcinoma | Total |
| Depth of invasion: MP, muscularis propria; SE, serosa exposed; SI, serosa infiltrating; SM, submucosa; SS, subserosa. Mode of infiltrative growth: INFα, the tumour shows expanding growth and a distinct border from the surrounding tissue; INFβ, this category is between INFα and INFγ; INFγ, the tumour shows infiltrating growth and an indistinct border from the surrounding tissue. Conclusive stage grouping: stage I, t1(M•SM)n0, t1n1, or t2 (MP•SS) no; stage II, t3(SE)no, t2n1, or t1n2; stage III, t4(SI)n0, t3n1, t4n1, t2n2, t3n2, t1n3, or t2n3; stage IV, t4n2, t3n3, or cases with direct invasion into surrounding organs, peritoneal dissemination, and/or liver metastasis. | |||
| Depth of invasion | |||
| SM | n=5 | n=4 | n=9 |
| MP | n=4 | n=1 | n=5 |
| SS | n=5 | n=4 | n=9 |
| SE | n=3 | n=4 | n=7 |
| SI | n=1 | n=0 | n=1 |
| Mode of infiltrative growth | |||
| INFα | n=5 | n=2 | n=7 |
| INFβ | n=12 | n=4 | n=16 |
| INFγ | n=1 | n=7 | n=8 |
| Lymph node metastasis | |||
| Present | n=10 | n=6 | n=16 |
| Absent | n=6 | n=6 | n=12 |
| Conclusive stage grouping | |||
| Stage I | n=7 | n=6 | n=13 |
| Stage II | n=3 | n=1 | n=4 |
| Stage III | n=5 | n=4 | n=9 |
| Stage IV | n=1 | n=1 | n=2 |
Immunohistochemistry
For immunohistochemistry, portions of tissue samples from the cancerous lesion, from unaffected mucosa, and from neonatal mucosa were sliced into sections approximately 5 mm thick within one hour of removal, immersed for about six hours in periodate/lysine/paraformaldehyde fixative, rinsed in graded sucrose/0.01M phosphate buffered saline (PBS; pH 7.4), embedded in optimal cutting temperature compound (Miles Inc, Elkhart, Indiana, USA), and stored at −80°C until sectioning on a cryostat. The primary antibodies used for immunochemistry were rabbit polyclonal antibodies against C3d and S protein (Dakopatts, Glostrup, Denmark), and monoclonal antibodies against C5b–9 (aE11; mouse IgG2a; Dako, Copenhagen, Denmark), CR1 (To5; mouse IgG1; Dako), MCP (J4–48; mouse IgG1; Immunotech, Marseille, France), DAF (1C6; mouse IgG1; Wako, Tokyo, Japan), and protectin (MEM-43; mouse IgG1; Serva, Heidelberg, Germany). Biotinylated affinity purified goat antirabbit IgG and horse antimouse IgG (Funakoshi, Tokyo, Japan) were used as secondary antibodies. Representative cryostat sections were stained using the avidin–biotin–peroxidase complex (ABC) technique. The primary antibodies were incubated overnight at 4°C. After washing in PBS, biotinylated affinity purified antibodies were incubated for one hour at room temperature. The sections were washed in PBS and incubated with the ABC reagent for one hour at room temperature, followed by 3,3′-diaminobenzidine (Dojin Chemicals, Kumamoto, Japan) as a chromogen. Finally, the slides were counterstained with methyl green. Negative control sections (PBS, non-immune rabbit serum, or mouse IgG (Dakopatts) instead of antibody) were run at the same time. Lymphoid germinal centres from the appendix of a patient who underwent right hemicolectomy as a result of ascending colon cancer were used as positive controls for each antibody tested. The staining intensity was estimated on a five point scale (0, ±, 1+, 2+, and 3+), and a score was obtained according to that intensity, as described previously.19 After immunostaining, representative periodate/lysine/paraformaldehyde fixed sections were used for immunoelectron microscopy. In brief, the cryostat sections, 10 μm thick, were incubated with primary antibody for 24 hours at 4°C. After washing, the sections were incubated with horseradish peroxidase conjugated F(ab′)2 fragments of affinity isolated rabbit antimouse IgG antibody and goat antirabbit IgG antibody (American Qualex, San Clemente, California, USA) for two hours at 4°C, and the staining was visualised with diaminobenzidine. After refixing with 1.25% glutaraldehyde for 30 minutes and 2% osmium tetroxide for two hours at 4°C, the specimens were dehydrated through graded alcohols and embedded in epoxy resin. Ultrathin sections were prepared using an ultramicrotome, and observed under an electron microscope. Three gastric specimens of histologically normal (uninvolved) mucosa were used for immunoelectron microscopy; one was obtained from fundic mucosa without intestinal metaplasia and gastric cancer, and two were obtained from pyloric mucosa with intestinal metaplasia and without gastric cancer.
Statistical analysis
Statistical analysis was performed using Statview II software (Abacus Concepts Inc, Berkeley, California, USA). Mean values were compared by the Kruskal-Wallis test and Scheffe's methods.
RESULTS
Expression of complement components and regulatory proteins in uninvolved gastric mucosa
The expression of complement components and regulatory proteins in uninvolved gastric mucosa obtained from patients with gastric cancer was examined. There were no significant differences in the staining intensities among the three different anatomical sites (upper third (n = 7), middle third (n = 5), and lower third (n = 5)), between pyloric gland mucosa (n = 9) and fundic mucosa (n = 8), between mucosae with (n = 9) and without (n = 8) intestinal metaplasia, and between mucosae with (n = 16) and without (n = 17) gastric cancer. In normal gastric epithelium, complement components C3d and C5b–9 and the regulatory protein S protein were deposited on basal cell surfaces. Their staining patterns were predominant in the upper parts of the foveolae. On immunoelectron microscopy it was confirmed that C3d, C5b–9, and S protein were deposited only on the basal cell membranes (table 2 ▶).
Table 2.
Ultrastructural localisation of complement components and regulatory proteins on the cell membrane of gastric cells
| Localisation on gastric cells | |||
| Complement components and regulatory proteins | Basal membrane | Lateral membrane | Luminal membrane |
| Uninvolved mucosa (n=3) | |||
| C3d | ++ | − | − |
| C5b–9 | + | − | − |
| MCP (CD46) | ++ | ++ | − |
| DAF (CD55) | − | − | + |
| S protein | ++ | − | − |
| Protectin (CD59) | + | − | + |
| Differentiated adenocarcinoma (n=3) | |||
| C3d | ++ | − | + |
| C5b–9 | + | − | ± |
| MCP (CD46) | ++ | ++ | − |
| DAF (CD55) | − | + | ++ |
| S protein | ++ | − | ± |
| Protectin (CD59) | ++ | + | ++ |
| Undifferentiated adenocarcinoma (n=1) | |||
| C3d | − | ||
| C5b–9 | − | ||
| MCP (CD46) | ++ (circumferential cell membrane staining) | ||
| DAF (CD55) | ++ (circumferential and/or luminal cell membrane staining) | ||
| S protein | − | ||
| Protectin (CD59) | − | ||
DAF, decay accelerating factor; MCP, membrane cofactor protein; −, negative; ±; occasionally positive; +, weakly positive; ++, strongly positive.
MCP was always expressed strongly and formed a homogeneous pattern in all normal tissues. MCP was expressed simultaneously on both the basal and lateral membranes, but very rarely on the luminal membrane. In neonatal mucosa DAF was negative, whereas in non-cancerous mucosa only focal expression of DAF on the luminal surface was seen. The ultrastructural localisation of DAF in non-cancerous mucosa confirmed this finding. Protectin expression was patchy in normal tissues. In the positively stained areas, it was expressed weakly on the luminal membrane and on the basal membrane. CR1 was always negative.
Expression of complement components and regulatory proteins in cancerous gastric mucosa
In differentiated adenocarcinomas, the staining patterns of C3d, C5b–9, and S protein were usually the same as in histologically normal tissues, but in a part of the differentiated adenocarcinomas, complement components C3d (n = 5) and C5b–9 (n = 2), and S protein (n = 3) were deposited not only on the basal cell surfaces, but also on the luminal surfaces, as shown by light microscopy (fig 1A,B ▶). Ultrastructural observations confirmed that these three antigens produced basal staining and that the staining was absent on the lateral membranes, although it was sometimes present on the luminal membranes (table 2 ▶). In undifferentiated adenocarcinoma, C3d and S protein were always negative. The staining intensity of C3d, C5b–9, and S protein revealed no significant correlation between the four different histological groups (table 3 ▶).
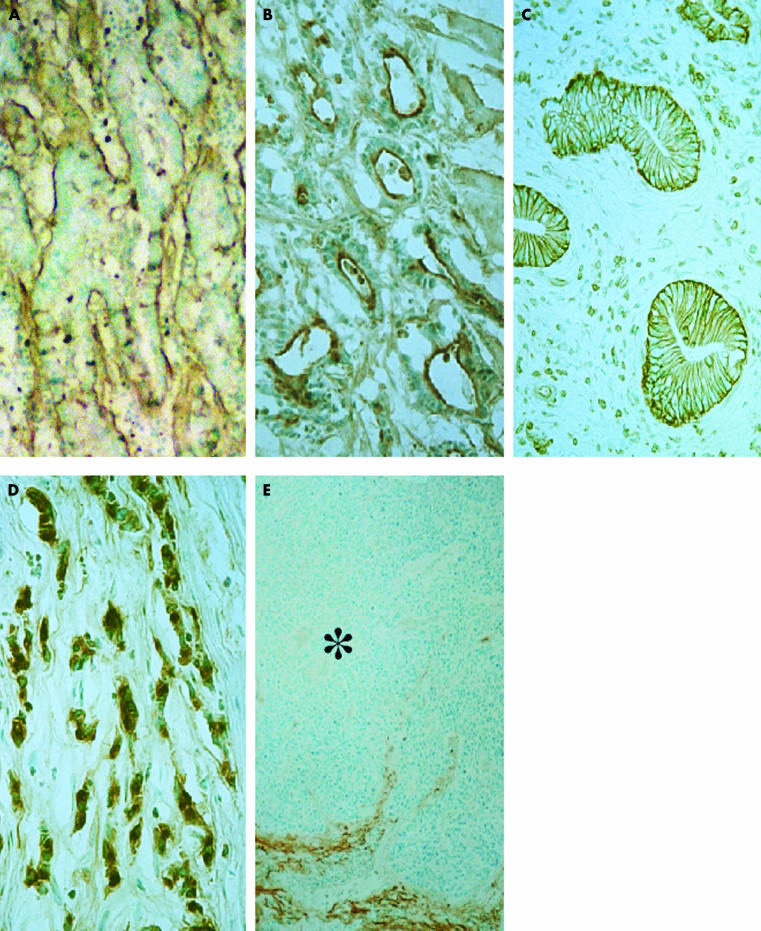
Figure 1.
Immunostaining of complement component C3d and regulatory proteins. (A) C3d in differentiated (tubular) adenocarcinoma. The positive reaction is limited to the basal surface. (B) S protein in differentiated (tubular) adenocarcinoma. The positive reaction is limited to the luminal surface. (C) Membrane cofactor protein (CD46) in differentiated (tubular) adenocarcinoma. Note the constant staining on the basolateral membrane, but not on the luminal surface. (D) Decay accelerating factor (DAF; CD55) in undifferentiated (poorly differentiated) adenocarcinoma with a diffusely infiltrative growth pattern (INFγ). Note the intracytoplasmic expression in almost all cancer cells. (E) DAF (CD55) in undifferentiated (poorly differentiated) adenocarcinoma with an expanding growth pattern (INFα). A cancer cell nest (asterisk) does not show the distinct positive reaction. Counterstained with methyl green; original magnification: A–C, ×640; D, ×980; E, ×320.
Table 3.
Staining intensity of complement components and regulatory proteins in gastric tissues
| Mean staining intensity (SD) | ||||||||
| Complement components and regulatory proteins | Neonatal mucosa | Uninvolved mucosa | Differentiated adenocarcinoma | Undifferentiated adenocarcinoma | ||||
| C3d | n=2 | 2.22 (0.51) | n=17 | 2.57 (0.82) | n=18 | 2.67 (1.04) | n=13 | 1.21 (0.93) |
| C5b–9 | n=2 | 0 | n=17 | 1.32 (0.49) | n=18 | 1.67 (0.63) | n=13 | 1.38 (1.23) |
| MCP (CD46) | n=2 | 5.11 (0.98) | n=17 | 5.75 (0.52) | n=18 | 5.41 (1.06) | n=13 | 5.31 1.11) |
| DAF (CD55) | n=2 | 0 | n=17 | 1.51 (0.87)** | n=18 | 3.78 (1.72)** | n=13 | 4.22 (1.35)** |
| CR1 (CD35) | n=2 | 0 | n=13 | 0 | n=9 | 0 | n=6 | 0 |
| S protein | n=2 | 1.22 (0.53) | n=17 | 1.59 (0.76) | n=18 | 2.13 (1.24) | n=13 | 0.75 (0.62) |
| Protectin (CD59) | n=2 | 0.93 (0.45) | n=17 | 2.81 (0.98)* | n=18 | 3.33 (1.29)** | n=13 | 1.11 (0.72)*,** |
CR1, complement receptor type 1; DAF, decay accelerating factor; MCP, membrane cofactor protein.
*p<0.05; **p<0.01.
The expression of MCP in malignant tissues was similar to that in normal tissues (fig 1C ▶), except in undifferentiated adenocarcinomas. In undifferentiated adenocarcinomas, which have few glandular structures, MCP was expressed over the entire cell surface. This pattern was consistent with the findings on immunoelectron microscopy (table 2 ▶; fig 2A ▶). There was no significant difference in the staining intensity of MCP between the four different histological groups (table 3 ▶).
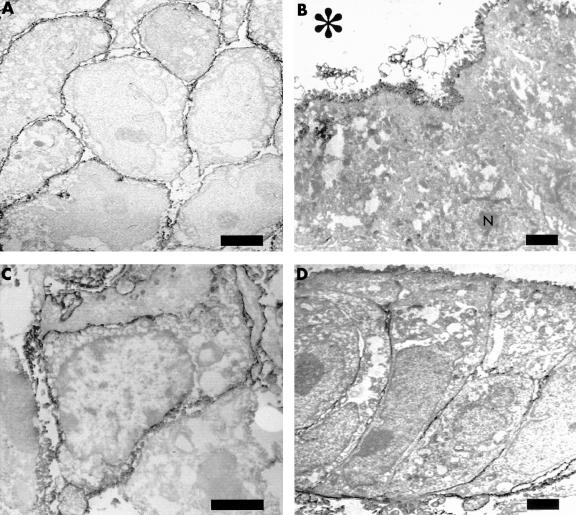
Figure 2.
Immunoelectron micrograph of regulatory proteins. (A) Membrane cofactor protein (CD46) in undifferentiated (poorly differentiated) adenocarcinoma. Unlike differentiated adenocarcinoma, the positive reaction is seen over the entire surface (bar, 1 μm; original magnification, ×4400). (B) Decay accelerating factor (DAF; CD55) in differentiated (papillary) adenocarcinoma. Note the positive reaction only on the luminal cell surface; N indicates the nucleus (bar, 1 μm; original magnification, ×3000). (C) DAF (CD55) in undifferentiated (poorly differentiated) adenocarcinoma. Unlike differentiated adenocarcinoma, the positive reaction is found over the entire surface (bar, 2 μm; original magnification, ×6000). (D) Protectin (CD59) in differentiated (tubular) adenocarcinoma. The strong reaction is found on the luminal and basal membrane, and the weak reaction on the lateral membrane (bar, 1 μm; original magnification, ×3000). The asterisk indicates the lumen of the cancer cells.
In differentiated adenocarcinomas, DAF was expressed on cell surfaces adjacent to the lumen, but occasionally the apicolateral cell surfaces were positive. In undifferentiated adenocarcinomas, there were two staining patterns, depending on the presence or absence of glandular structures. When ducts were present, DAF was expressed on their luminal sides, and when they were absent, DAF was expressed over the entire surface. In addition, in one signet ring cell carcinoma, DAF was expressed in the cytoplasm. The ultrastructural localisation of DAF corresponded approximately with the findings on light microscopy. In differentiated adenocarcinoma, it was apparent on the luminal cell membranes (fig 2B ▶), and often on the lateral cell membranes. In undifferentiated adenocarcinoma DAF was expressed over the entire surface (fig 2D ▶).
The staining intensities of DAF in both differentiated and undifferentiated adenocarcinomas were significantly higher than in non-cancerous tissues (table 3 ▶). There was no significant difference in the staining intensity of DAF between differentiated and undifferentiated adenocarcinomas, or between the four tumour staging grades. However, there were significant differences according to the mode of infiltrative growth of the gastric cancers. The staining intensity of DAF in gastric cancers showing a diffusely infiltrating growth pattern (INFγ; mean value of staining intensity, 3.69; SD, 1.69; n = 8; fig 1D ▶) was significantly higher than that seen in gastric cancers showing an expanding growth pattern (INFα; mean value of staining intensity, 1.73; SD, 1.39; n = 7; p < 0.05; fig 1E ▶). The staining intensity of gastric cancers showing the intermediate mode (INFβ; mean value of staining intensity, 4.32; SD, 1.37; n = 16) was similar to that of INFγ, but was significantly higher than that of INFα (p < 0.01). There were no significant differences in the staining intensities of other complements factors and regulatory proteins in relation to the different clinicopathological parameters (depth of invasion, mode of infiltrative growth, lymph node metastasis, and stage grouping).
In differentiated adenocarcinomas, protectin was expressed strongly on the luminal membrane, as in normal tissues. In undifferentiated adenocarcinomas it was almost absent. Unlike normal (non-neoplastic) gastric epithelium, differentiated adenocarcinomas strongly expressed protectin on both the luminal and basal membranes (fig 2D ▶). The lateral membranes were also weakly stained (table 2 ▶). The staining intensity of protectin in undifferentiated adenocarcinomas was significantly lower than in both normal gastric epithelium and differentiated adenocarcinomas (table 3 ▶).
CR1 was found on the surfaces of cells in the non-neoplastic lymphoid germinal centres of both benign and malignant tissues, but was not expressed in any of the normal epithelial or cancer cells.
DISCUSSION
We investigated the expression of complement components and regulatory factors on gastric cancer cells, and their localisation sites on the cell membrane, by immunohistochemistry and immunoelectron microscopy. We confirmed that complement activation measured as deposition of C3d and C5b–9 occurs in both histologically normal gastric mucosa and cancer tissue, together with the presence of complement regulating factors that protect cells from complement mediated cytotoxicity. Interestingly, the expression patterns and staining intensities of some of the regulatory factors varied with the histology of the cancer tissues.
S protein, which is a serum protein that binds to nascent C5b–7/8 in serum, was present on the basal surface of normal gastric epithelium, as were C3d and C5b–9, indicating complement activation.20 Although complement components are present only partially in a few normal tissues, for example thyroid tissues and glomeruli,1,14 our study has shown that C3d and C5b–9 are deposited in histologically normal gastric epithelium. The staining patterns of these three antigens show that they are located predominantly in the upper parts of the crypts. These data indicated that complement is partly activated under normal circumstances. This could be the result of the continuous antigenic stimulation (by microorganisms and/or immune complexes) that the gut undergoes. Further investigations are needed to clarify this phenomenon.
In differentiated adenocarcinomas, the staining patterns of C3d, C5b–9, and S protein were almost identical to those in normal tissues. Interestingly, in several differentiated adenocarcinomas, they were deposited not only on the basal cell surfaces but also on the luminal ones, suggesting that in invasive carcinoma complement deposits on both surfaces, allowing cancer cells to be protected from complement mediated cytotoxicity by the expression of S protein.
“In contrast to the other three complement regulating factors, DAF staining intensities in both differentiated and undifferentiated adenocarcinomas were significantly higher than in normal epithelium”
MCP is a cofactor for factor I mediated cleavage of C4b/C3b, but it does not accelerate the decay of the C3/C5 convertases.21,22 The presence of antibodies directed against the surface antigens of tumour cells would activate the complement system through the classical pathway.23 In previous studies, it has been reported that MCP is ubiquitous on most human nucleated cells exposed to blood, including the epithelial cells of the normal gastric gland.24 In our present study, MCP was expressed strongly and ubiquitously in all benign and malignant gastric epithelial cells. Ultrastructural observations demonstrated that MCP was consistently present on both the basal cell border and the lateral membrane, but not on the luminal surface of normal gastric epithelial or differentiated adenocarcinoma cells. In contrast, in undifferentiated adenocarcinomas, it was present over the entire cell surface. These phenomena may reflect the particular need for regulators of complement activation on cells exposed to plasma.
Protectin is normally distributed widely over the membranes of human blood cells, vascular endothelial cells, middle ear mucosal cells,25 and certain cancer cells.13,14 Koretz et al studied protectin in colorectal carcinomas.13 They found that the expression of CD59 in these carcinomas correlated with their differentiation grade and the stage of the disease—carcinomas exhibiting a low differentiation grade and those that had already metastasised at the time of surgery lacked protectin in the neoplastic compartment significantly more often. The first of these statements agrees with our data but the second does not. It is unclear why protectin expression in undifferentiated adenocarcinomas was lower than in other types, but one reason might be the presence or absence of glandular structures, because in positive reactions protectin was strongly expressed on the luminal cell surface.
DAF acts only within the surface membrane of cells. It is strictly an intrinsic membrane inhibitor. DAF is present on the surface of the epithelial surface of the gastrointestinal mucosa, exocrine glands, and other glands.1 A human monoclonal antibody SC-1 against an isoform of CD55/DAF induces specific apoptosis of gastric carcinoma cells both in vitro and in an experimental in vivo system.16 Our present study showed that DAF was expressed on the luminal surface and occasionally on the lateral surface in both normal and malignant gastric epithelial cells. Of particular note, in contrast to the other three complement regulating factors, DAF staining intensities in both differentiated and undifferentiated adenocarcinomas were significantly higher than in normal epithelium. Furthermore, DAF expression in diffusely infiltrating carcinomas (INFγ) was higher than in expanding carcinomas (INFα). In addition, in the intermediate mode (INFβ) DAF expression was similar to that in the diffusely infiltrating mode. It may be inferred that diffusely infiltrating cancer cells can easily be exposed to complement mediated cytotoxicity compared with expanding types.
Our present study found that four complement regulating factors (S protein, MCP, protectin, and DAF) are expressed on gastric cancer cells, and determined their ultrastructural localisation on the cell membrane.
Take home messages.
The complement regulatory proteins, S protein, decay accelerating factor (DAF), membrane cofactor protein (MCP) and protectin were expressed on gastric cancer cells, although CR1 was not
The staining intensity of DAF was significantly higher in both differentiated and undifferentiated adenocarcinomas than in histologically normal gastric epithelium
In addition, the staining intensity of DAF was greater in gastric cancers showing a diffusely infiltrating growth pattern than in those that showed an expanding growth pattern
These data indicate that DAF may play a role in cancer cell infiltration and resistance in tumour cells
Abbreviations
ABC, avidin-biotin-peroxidase complex
CR1, type 1 complement receptor
DAF, decay accelerating factor
MCP, membrane cofactor protein
PBS, phosphate buffered saline
REFERENCES
- 1.Morgan BP, Harris CL. Complement regulatory proteins. San Diego: Academic Press, 1999.
- 2.Brasoveanu LI, Fonsatti E, Visintin A, et al. Melanoma cells constitutively release an anchor-positive soluble form of protectin (sCD59) that retains functional activities in homologous complement-mediated cytotoxicity. J Clin Invest 1997;100:1248–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nielsen CH, Matthiesen SH, Lyng I, et al. The role of complement receptor type 1 (CR1, CD35) in determining the cellular distribution of opsonized immune complexes between whole blood cells: kinetic analysis of the buffering capacity of erythrocytes. Immunology 1997;90:129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergelson JM, Mohanty JG, Crowell RL, et al. Coxsackievirus B3 adapted to growth in RD cells binds to decay-accelerating factor (CD55). J Virol 1995;69:1903–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong TC, Yant S, Harder BJ, et al. The cytoplasmic domains of complement protein CD46 interact with multiple kinases in macrophages. J Leukoc Biol 1997;62:892–900. [DOI] [PubMed] [Google Scholar]
- 6.Pasch MC, Bos JD, Daha MR, et al. Transforming growth factor-β isoforms regulate the surface expression on membrane cofactor protein (CD46) and CD59 on human keratinocytes. Eur J Immunol 1999;29:100–8. [DOI] [PubMed] [Google Scholar]
- 7.Zhang H, Yu J, Bajwa E, et al. Targeting of functional antibody–CD59 fusion proteins to a cell surface. J Clin Invest 1999;103:55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bjφrge L, Vedeler CA, Ulvestad E, et al. Expression and function of CD59 on colonic adenocarcinoma cells. Eur J Immunol 1994;24:1597–603. [DOI] [PubMed] [Google Scholar]
- 9.Simpson KL, Jones A, Norman S, et al. Expression of the complement regulatory proteins decay accelerating factor (DAF, CD55), membrane cofactor protein (MCP, CD46) and CD59 in the normal human uterine cervix and in premalignant and malignant cervical disease. Am J Pathol 1997;151:1455–67. [PMC free article] [PubMed] [Google Scholar]
- 10.Varsano S, Rashkovsky L, Shapiro H, et al. Human lung cancer cell lines express cell membrane complement inhibitory proteins and are extremely resistant to complement-mediated lysis; a comparison with normal human respiratory epithelium in vitro, and an insight into mechanism(s) of resistance. Clin Exp Immunol 1998;113:173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu J, Caragine T, Chen S, et al. Protection of human breast cancer cells from complement-mediated lysis by expression of heterologous CD59. Clin Exp Immunol 1999;115:13–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koretz K, Bruderlein S, Henne C, et al. Decay-accelerating factor (DAF, CD55) in normal colorectal mucosa, adenomas and carcinomas. Br J Cancer 1992;66:810–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koretz K, Bruderlein S, Henne C, et al. Expression of CD59, a complement regulator protein and a second ligament of the CD2 molecule, and CD46 in normal and neoplastic colorectal epithelium. Br J Cancer 1993;68:926–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamakawa M, Yamada K, Tsuge T, et al. Protection of thyroid cancer cells by complement-regulatory factors. Cancer 1994;73:2808–17. [DOI] [PubMed] [Google Scholar]
- 15.Bjφrge L, Hakulinen J, Wahlström T, et al. Complement-regulatory proteins in ovarian malignancies. Int J Cancer 1997;70:14–25. [DOI] [PubMed] [Google Scholar]
- 16.Hensel F, Hermann R, Schubert C, et al. Characterization of glycosylphosphatidylinositol-linked molecule CD55/decay-accelerating factor as the receptor for antibody SC-1-induced apoptosis. Cancer Res 1999;59:5299–306. [PubMed] [Google Scholar]
- 17.Hakulinen J, Meri S. Complement-mediated killing of microtumors in vitro. Am J Pathol 1998;153:845–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Japanese research society for gastric cancer. Japanese classification of gastric carcinoma, 1st ed. Tokyo: Kanehara Publishing Co, 1999.
- 19.King RJB, Coffer AI, Gilbert J, et al. Histochemical studies with a monoclonal antibody raised against a partially purified soluble estradiol receptor preparation from human myometrium. Cancer Res 1985;45:5728–33. [PubMed] [Google Scholar]
- 20.Niculescu F, Rus HG, Retegan M, et al. Persistent complement activation on tumor cells in breast cancer. Am J Pathol 1992;140:1039–43. [PMC free article] [PubMed] [Google Scholar]
- 21.Wang G, Liszewski MK, Chan AC, et al. Membrane cofactor protein (MCP; CD46): isoform-specific tyrosine phosphorylation. J Immunol 2000;164:1839–46. [DOI] [PubMed] [Google Scholar]
- 22.Devaux P, Christiansen D, Fontaine M, et al. Control of C3b and C5b deposition by CD46 (membrane cofactor protein) after alternative but not classical complement activation. Eur J Immunol 1999;29:815–22. [DOI] [PubMed] [Google Scholar]
- 23.Kojima A, Iwata K, Seya T, et al. Membrane cofactor protein (CD46) protects cells predominantly from alternative complement pathway-mediated C3-fragment deposition and cytolysis. J Immunol 1993;151:1519–27 [PubMed] [Google Scholar]
- 24.Johnstone RW, Loveland BE, McKenzie IFC. Identification and quantification of complement regulatory CD46 on normal human tissues. Immunology 1993;79:341–7. [PMC free article] [PubMed] [Google Scholar]
- 25.Närkiö-Mäkelä M, Jero J, Meri S. Complement activation and expression of membrane regulators in the middle ear mucosa in otitis media with effusion. Clin Exp Immunol 1999;116:401–9. [DOI] [PMC free article] [PubMed] [Google Scholar]