Abstract
Purpose
Cryopreserved homografts for valve replacement surgeries face a major problem regarding their durability after implantation and decellularized pulmonary heart valves have raised as potential new generation substitute for these surgeries. The present study aims to document the work performed for the safe implementation in public tissue banks of a new decellularization method for human pulmonary heart valves, based on previous risk evaluation.
Methods
After assessing new preparation method associated risks, using EuroGTP-II methodologies, an extensive array of in vitro studies were defined to validate the new technique, mitigate the risks and provide quality and safety data.
Results
Initial evaluation of risks using EuroGTP II tool, showed Final Risk Score of 23 (high risk), and four studies were devised to mitigate identified risks: (i) tissue structure integrity; (ii) cell content; (iii) microbiological safety; and (iv) cytotoxicity evaluation in final tissue preparation. Protein quantification, mechanical properties, and histological evaluation indicated no tissue damage, reducing implant failure probability, while cellular content removal demonstrated a 99% DNA removal and microbiological control ensured contamination absence. Moreover, in vitro results showed no cytotoxicity. Risk re-evaluation indicated a risk reduction to moderate risk (Final Risk Score = 10), suggesting that further evidence for safe clinical use would be needed at pre-clinical in vivo evaluation to mitigate remaining risks.
Conclusions
The studies performed and reviewed bibliography were able to significantly reduce the original level of risk associated with the clinical application of this homograft’s preparation. However, additional in vivo studies and tissue stability tests are still necessary to address the remaining risks associated with reagents’ effect on extracellular matrix and storage conditions, which could influence implant failure, before the clinical evaluation procedures can be implemented to determine the efficacy and safety of the new decellularized heart valves.
Keywords: Pulmonary valve, decellularization, extracellular matrix, risk assessment, EuroGTP II
Graphical Abstract
Introduction
Valve replacement is a widely performed surgical procedure for treating diseased heart valves that cannot be managed through other treatment options. Mechanical valves were first introduced in the 1940s, while xenografts and homografts were successfully implanted in humans in the 1960s. Human allogenic heart valves started to be used as a best available substitute for dysfunctional heart valves, due to their optimal hemodynamical characteristics, their elevated resistance to infections and longer durability compared to the valves of animal origin.1 Initially, heart valves were freshly implanted within 6–8 weeks2 after being harvested and stored in the antibiotic cocktail,3 but the establishment of the cryopreservation technique assured the increase of safety and availability of the homograft’s.4 It is widely accepted that immune reactions towards the donor cells trigger the inflammatory response that concludes in the deterioration and calcification of the graft; therefore, this removal of cells pretends to decrease the immunogenicity of the tissue and barely promote its degradation. Specifically, for young patients cryopreserved valves are not a definitive alternative to assure correct functioning of the valve at long term, as the high activity of the immune system in these patients leads to the infiltration of monocytes into the cryopreserved homograft matrix degrading collagen fibers and consequently calcifying the tissue becoming dysfunctional5 and requiring valve replacement reinterventions, which carry associated risks. Decellularization is a technique that aims to completely eliminate, or at least reduce into a negligible level, the cellular components of the donor, while preserving the extracellular matrix of the tissue using a combination of reagents and/or physical and mechanical methods.6,7 Physical, chemical and/or enzymatic procedures are performed to ensure that the DNA is below 50ng/mg of dry tissue, while assuring the maintenance of the extracellular matrix; this threshold is reported by Crapo et al, and is commonly considered the limit for decellularization, that should be confirmed by histology and the absence of nucleic acid fragments longer than 200 base pairs.7 As a result, once the graft is transplanted, there is a negligible, but not inexistent immune response in the recipient.8 These methods have to ensure that the quality and safety of the graft is preserved. Over the last 15 years, several studies with various preparation methods and promising results have been published with decellularized valves.9–14 The main advantage of these allografts, apart from a good mechanical stability, is that they were supposed to avoid generating an immunological response, avoiding the degeneration of the tissue and enlarging their durability. Moreover, different studies showed successful early and mid-term performance of decellularized pulmonary valve (dPV) allografts without calcification.15–19 In addition, there are some studies referring to the incipient colonization of recipient cells into the homograft supporting of the tissue homeostasis and decreasing the passive deterioration.5 Furthermore, other studies highlight preliminary findings showing adaptive growth of the pulmonary valve annulus through somatic growth.20–22
European Tissue Establishments (TE) must comply with high-quality and safety standards according to the requirements of the European Union Tissues and Cells Directives (EUTCD) and the best practices defined by the European Directorate for the Quality of Medicines & HealthCare (EDQM) to ensure a high level of health protection.23 Cardiovascular tissue banking programs were established between the end of the 1960s and the 1990s all around Europe24 and their activities are constantly evolving in search of better care treatment for patients who require this type of therapy. The main aim of a TE is to give the optimal solution to the surgeons for repair and/or replacement of diseased valves and, for this reason, the continuous process improvements and new developments hold a paramount level of interest and significance. However, the development of new tissue preparations may result in some risks due to changes in the preparation process or associated with novel clinical application or indication. While allogenic heart valves decellularization methods have not previously been developed in our TEs, these types of tissues have been prepared and clinically used for a long time,25–28 and have demonstrated safety and effectiveness when implanted in patients. Moreover, technical specifications outlined in the Tissue and Cell Monographs defined by the EDQM, and categorize decellularized pulmonary valves as a standard therapy.23
The Good Practices for demonstrating safety and quality through recipient follow-up (EuroGTP II) methodology emphasizes the criticality of assessing potential risks associated with significant changes in the procedures, tissue preparations, or clinical application.29,30 It proposes a systematic methodology, utilizing a risk-based mechanism and an Interactive Assessment Tool (IAT), to define pre-clinical and clinical evaluations of Substances of Human Origin (SoHO)31,32 required for designing studies to ensure the safety and quality of new tissue preparations. Since 2019, European TEs have utilized the EuroGTP II methodology to identify, assess, and mitigate risks linked to the advancement of new preparation techniques and modifications to existing processes.33,34 This standardized approach has enabled the quantification of risk levels associated with innovative SoHO, the implementation of uniform strategies to minimize these risks, and the establishment of appropriate clinical assessments to demonstrate safety and efficacy.35
The current investigation addresses the necessary steps for safely implementing a new decellularization protocol for pulmonary valve (PV) into our TE’s regular procedures. These steps include assessing novelty, conducting risk evaluations, designing and executing studies to mitigate identified risks. We included the results of the studies performed to develop an efficient and efficacious decellularization process for PV, which allows the removal of the cellular content, maintaining the composition and structure of the extracellular matrix. The novel preparation method consists of a two-step procedure that uses gentle reagents to remove almost completely donor’s DNA, while maintaining the structure and function of the homograft. It allows for minimal tissue handling and manipulation, and its integration into the routine activities of our TE maintaining aseptic procedures, thus makes it an affordable therapy, suitable for the public healthcare system.
Materials and Methods
Risk Assessment
The EuroGTPII risk-assessment methodology has been described elsewhere.36 Briefly, it consists of three steps: the new product is characterized to properly evaluate the novelty (Step 1); the risks associated with the novelty are identified and quantified through a risk assessment (Step 2). The results of this assessment provide a Final Risk Score that was used to define the extent of the studies (Step 3) required to safely implement the novel therapy or technique. In this work only, the in vitro studies required to safely implement tissue preparation into a clinical setting are addressed. The use of EuroGTPII methodologies is supported by the use of an IAT (http://tool.goodtissuepractices.site).
Validation and Pre-Clinical Studies
Based on the results of the risk assessment, the following pre-clinical validation studies were proposed and performed.
Donor Selection Criteria: For tissue banking purposes, heart-beating (brain death), non-heart-beating, and exitus donors are screened for heart valve donation. A comprehensive evaluation is conducted to identify any contraindications, including medical and social history, serological and microbiological tests for transmissible diseases, physical examination, and autopsy findings when applicable. Mandatory serology tests include HIV, hepatitis B and C, syphilis, HTLV, and nucleic acid testing for HIV, hepatitis B and C, and hepatitis E. Additional tests, such as for Trypanosoma cruzi or West Nile Virus, may be included depending on the donor’s origin, travel history, and the epidemiological context.37 These practices aligned with the EU standards for donation of substances of human origin defined in the EDQM Guide, European and national regulations.
Tissue retrieval: Hearts were procured from 24 human cadaveric donors after obtaining informed consent from their relatives. They were retrieved opening the thoracic cavity via sternotomy preserving the aortic arch and pulmonary branches as long as possible,37 placed in a sterile container with transport media and refrigerated until processing. After processing, those PV not acceptable for clinical implantation, but suitable for this study and with informed consent for research purposes, underwent the process of decellularization or where used as standard fresh or cryopreserved control tissues. The 24 donors were divided in 4 groups of analysis (fresh VP, cryopreserved VP, fresh decellularized VP and cryopreserved decellularized VP). Ethics committee approval was issued by the Hospital Vall d’Hebron (Barcelona) Research Ethics Committee for Medicinal Products (RECm); PR (BST) 315/2019. Donor screening included, but was not limited to, a review of complete social and medical history, physical examination of the donor, serological and microbiological testing during retrieval according to national directives, and autopsy findings if applicable, as well as any other relevant information.
Macroscopic evaluation: Before the decellularization process, the tissue was inspected following the standard procedure of our TEs looking for atheroma, calcifications, fenestrations or any features that could invalidate the tissue for the in vitro assays described in the following sections. After decellularization, tissue was evaluated again to ensure that the process did not affect the structure macroscopically.
- Quantitative and qualitative methods.
- Residual DNA quantification: Biopsies of the arterial wall, leaflets, and myocardium were taken from six independent donors per group (N). Each tissue sample was evaluated in triplicate (n). Briefly, the samples were lyophilized before DNA extraction that was obtained using a commercial kit (QIAamp DNA Mini Kit – Quiagen, 51304). Briefly, 10 mg of lyophilized decellularized tissue or 5mg of lyophilized native tissue were weighted, lysed in 200 µl ATL buffer with Proteinase K for 24h at 56°C, and the resulting solution passed through the affinity column. Two serial 200 uL elutions were used to elute the DNA from the columns. The amount of DNA was quantified by spectrophotometry using the PicoGreen commercial kit (Thermo Fisher, P11496) and measured on the Triad Multi-Mode Microplate Reader (Dynex Technologies). The tissue was considered decellularized if its DNA content was below 50 ng /mg dry tissue.
- Extracellular matrix biomolecules quantification: Biopsies of the arterial wall, leaflets, and myocardium were taken from six independent donors per group (N). Each tissue sample was evaluated in duplicate (n). The quantification was performed by colorimetric testing of total collagen and elastin, as structural proteins, and of glycosaminoglycans (GAGs) as carbohydrate structure. For all tests, 5 mg of lyophilized tissue (from both native, as control, and decellularized samples) were weighed, and quantification was performed using the following kits according to manufacturer’s instructions: the Sircol™ Soluble Collagen assay kit (Bicolor life science assays, S1000) for total collagen (acid-soluble and pepsin-soluble) testing; the Fastin™ Elastin assay kit (Bicolor life science assays, F2000) for elastin (α-elastin after acid treatment) testing; and the Blyscan™ Glycosaminoglycan assay kit (Bicolor life science assays, B1000) for total glycosaminoglycans (GAGs; sulphated GAG content after papain extraction). All of them are dye-binding methods and absorbance was read with an Epoch microplate spectrophotometer (Biotech) at 570 nm (collagen), 513 nm (elastin), and 656 nm (GAGs). The results are presented as μg of specific protein/mg dry weight tissue.
- Histological structure: 2 histological sections of approximately 1×0.5 cm of the arterial wall and the myocardium were performed for 3 independent donors. The samples were fixed in a 4% formaldehyde solution at 4°C ON. Subsequently, two washes were performed with phosphate-buffered saline (PBS) and the samples were preserved in 30% EtOH. Each sample was dehydrated, embedded in paraffin and cut transversely at 5 µm intervals, creating sections that were transferred into slides. The sections were stained with Hematoxylin-Eosin, Masson’s Trichrome and Verhoeff-Van Gieson staining. Images were taken from each slide using the bright-field microscope Axio Scope A1 (Zeiss) with the AxioCam MRc5 camera.
-
Biomechanical properties: Two different assays were performed to analyze the biomechanical properties of the decellularized pulmonary valves: uniaxial biomechanical assay and hydrodynamic assay.
Uniaxial biomechanical assay: 2 arterial wall samples from six independent donors (one longitudinal and one transversal; Figure 1b) were subjected to increasing uniaxial stress until breakage by means of a universal tensile strength tester (Instron 3366), which measures the resistance of the tissue to a controlled force. Bone-shaped samples measuring 2×4 cm were prepared and secured as previously described,38 to test 2 cm of the tissue (Figure 1d). The specific thickness of each sample was measured using a micrometer before stretching. The samples were preconditioned at a speed of 12 mm/min until reaching a load of 0.5 N, which was defined as the unstretched length (Lo). Subsequently, the samples were stretched at a speed of 12 mm/min until breakage (Figure 1c), and the mechanical properties of each sample were determined from the stress-strain (σ-ε) curve. The mechanical properties analyzed included maximum load (N), Young’s modulus (N/mm), stiffness (N/mm²), and elongation at maximum load (%).
- Hydrodynamic assay: Two different tests were performed using 3 fresh decellularized PV and 3 cryopreserved PV as control.
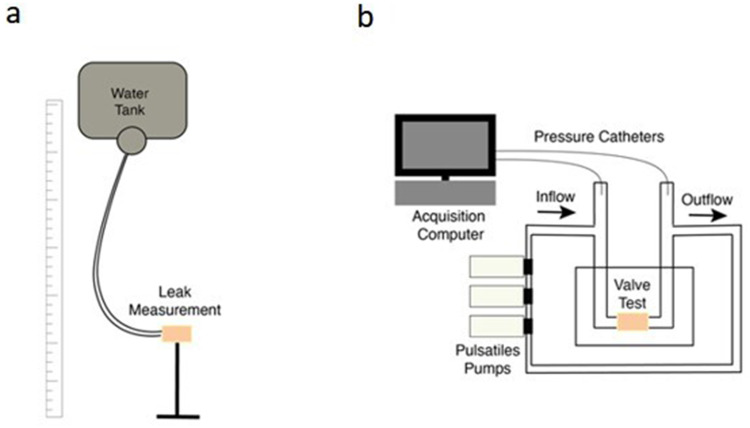
- I. Hydrodynamic behavior I: Complete pulmonary valves were used to perform a competence test was performed under static pressure to check each valve’s occlusion performance under physiological pressures. The proximal part of the pulmonary artery, of the valve to be tested, was adjusted to a semi-rigid tube connected to an elevated water tank. Valve closing competence was assessed by measuring the leakage rate for 1 minute at several heights mimicking physiological pressures (Figure 2).
- II. Hydrodynamic behavior II: Complete pulmonary valves were used to evaluate valve behavior under physiological (pulsatile flow) conditions. The valves were placed into a bioreactor and its ends were adjusted to a closed, recirculating, water circuit. This circuit consists of 3 pulsatile pumps connected in series, controlled by a computer capable of operating at different physiological regimes and a feed tank preheated to approximate blood temperature. We tested the valves at 60, 90 & 120 bpm, ensuring a constant flow rate around 5 L/min while monitoring the pressure range to mimic physiological pressure drops in the valves. 2 Millar 5F catheters were positioned at the inlet and outlet of the valves to measure upstream / downstream pressures. Signals were captured using a Texas Inc acquisition card and processed using custom algorithms written in MATLAB (Figure 2).
Cytotoxicity assay: The cytotoxicity study was performed following the cell culture model defined in the directive of ISO 10993–5. The method evaluates indirectly whether, after decellularization protocol, it remains any residual chemical components in the matrix, which could produce a cytotoxic effect. The extract of the decellularized arterial wall and myocardium biopsies were prepared, after the washing protocol (5 serial 2-minute washes with 500 ml of 0.9% NaCl), as described in ISO 10993–12. The extracts were prepared with 100 mg of tissue submerged in DMEM medium for 24 h at 37 °C. The extract was loaded on a monolayer of 3T3-J2 subconfluent cells seeded 24 h previously at a cell density of 20,000 cells/well. Finally, the viability assay was performed after 24 h using WST-1 reagent (Abcam), measuring the absorbance at 480 nm. The calculations were expressed as a percentage with respect to a negative control of cytotoxicity, the cell culture medium. The assay also includes a positive cytotoxicity control, a latex extract. Tissue is considered biocompatible and non-cytotoxic if obtained cell viability is equal to or greater than 70%.
Microbiological assessment: Microbiological tissue samples were taken in each step of the decellularization protocol and included in thioglycolate broth media (28410, Biomerieux) for aerobic/anaerobic growth or liquid samples from transport media are inoculated in BD BACTEC™ PLUS-Aerobic/F Medium (BD Bioscience, 442192) and BD BACTEC™-Lytic/10 Anaerobic/F Medium (BD Bioscience, 442265) for aerobic and anaerobic growth and fungi detection. Solid samples in thioglycollate tubs were incubated for 30 days at 37°C and its turbidity is checked daily; when samples presumed positive were sub-cultured in aerobic and anaerobic agar plates for microorganism identification. Liquid samples were inoculated in blood culture bottles BD Bactec™ System and incubated at 37°C for 5 to 7 days. Through fluorescent technology, the system detects the presence of microbial growth within the bottles. If no changes were identified after 5 days, the culture was considered negative. If a shift in the medium was detected by infrared spectrophotometry monitor, indicating the generation of carbon dioxide, the system was issued a positive alert meaning there was microorganism growth. In such instance sub-cultured in aerobic and anaerobic agar plates was carried out for microorganism identification.
Statistical analysis: The PRISM software version 5.00 (GraphPad Software, San Diego CA, USA, GraphPad®) was used for statistical analysis. Results are presented as the mean ± standard deviation (MD ± SD) or median and interquartile range obtained from five independent donors per group and three independent values per donor (see Supplemental Table 1). The non-parametric two-tailed Mann-Whitney test was used and p values less than 0.05 (p<0.05) were considered statistically significant.
Figure 1.
Samples processing to perform uniaxial mechanical assay. (a) Decellularized pulmonary valve (b) longitudinal and transversal bone-shape samples. (c) bone-shape samples after stretching and (d) schematic diagram of bone-shaped-samples Anchorage on tensile strength tester before stretching.
Figure 2.
Schematic view of test bench for hydrodynamic analysis. (a): the height of the water column is modified to adjust it to different physiological pressures for the leaking test. (b): Sketch of the closed flow circuit to assess the hemodynamic pressure response of the valves.
Results
Initial Risk Assessment
The first step of EuroGTP-II risk-assessment methodology was to evaluate the novelty of the new product, answering the proposed questions. The novelty involved with this new tissue preparation is related to the new processing method required to obtain dPV, which will need to be validated according to the specifications of the new tissue.
After novelty evaluation, the risks and their associated consequences for the recipients were evaluated. The rationale and scoring obtained are recorded in Table 1, as a result of the algorithm used in the interactive tool. This initial assessment indicated a high level of risk (Final Risk Score = 23), suggesting that more evidence is needed to support safe and effective use of this new tissue preparation.
Table 1.
Assessment of Risk Associated with the Implementation and Clinical Use of dPV
| Risk factors | Does it apply? | Justification | Risk consequences | P | S | D | PR | RR (%) | Risk score | |
|---|---|---|---|---|---|---|---|---|---|---|
| Preparation process | Processing and environment | Yes | The main risk of decellularization process is the probability of not accomplishing the complete removal of the cellular content, which could lead to unwanted immunogenicity. | Unwanted immunogenicity | 3 | 1 | 5 | 15 | 0 | 15 |
| Considering the duration and the complexity of the decellularization procedure, we consider that the risk of tissue contamination is possible. | Disease transmission | 3 | 3 | 2 | 18 | 0 | 18 | |||
| Reagents | Yes | The decellularization process requires several additional reagents, which have been priory used by BTB for other procedures. However, it is possible that the reagents used may damage the structure of the tissue, increasing the risk of implant failure in the recipient. | Implant Failure | 3 | 4 | 4 | 48 | 0 | 48 | |
| Adding different solutions during the preparation process, may increase the risk of tissue contamination during processing, comparing with the current processing protocol. | Disease transmission | 2 | 2 | 2 | 8 | 0 | 8 | |||
| We consider that the residual concentrations of all the reagents used in the procedure may produce an adverse effect in the recipient. In terms of carcinogenicity, considering that the reagents includes an authorized drug, we considered it unlikely. | Toxicity / Carcinogenicity |
3 | 2 | 5 | 30 | 0 | 30 | |||
| Reliability of microbiology testing | Yes | The reagents used may affect the reliability of testing at different stages of processing | Disease transmission |
1 | 2 | 2 | 4 | 0 | 4 | |
| Storage conditions | Yes | The storage method may damage the structure of the decellularized tissue. | Implant Failure | 3 | 4 | 3 | 36 | 0 | 36 | |
| Preliminary Score (Σ individual risk scores) | 159 | |||||||||
| Combined Risk Value ((Preliminary Score x Highest Possible Score)/((Max S × Max P × Max D) × Number of applicable risk consequences) = (159 x 4500)/(100 x 7) | 1022 | |||||||||
| Final Risk Score ((Combined Risk Value ×100)/ Highest Possible Score) | 23 | |||||||||
Notes: Adapted from the EuroGTP-II guidelines (EuroGTP-II, 2019).36 Green: Low risk level, Orange: Moderate risk level, Red: high risk level.
Abbreviations: D, detectability; P, probability; PR, potential risk; RR, risk reduction; S, severity.
In vitro Pre-Clinical Validation Experiments
For the mitigation of the potential risk consequences identified in Table 1, a set of specific in –vitro studies for process validation were proposed, which were divided in the following categories:
Evaluation of tissue structure: macroscopic evaluation, main extracellular matrix (ECM) components quantification, mechanical assay and histology, for the mitigation of implant failure due to structure damage related to the reagents. Fresh and cryopreserved decellularized PV were compared to fresh and cryopreserved PV.
Evaluation of cell content after decellularization: DNA quantification, for the mitigation of unwanted immunogenicity due to insufficient removal of cellular content.
Evaluation of the microbiology during the process, for the mitigation of disease transmission due to inclusion of different solutions and/or the duration and complexity of the procedure.
Evaluation of final product cytotoxicity, for the mitigation of toxicity/carcinogenicity related to the eventual residual concentrations of the used reagents.
Evaluation of Tissue Structure: Macroscopic Evaluation, Main ECM Components Quantification, Mechanical Assay and Histology
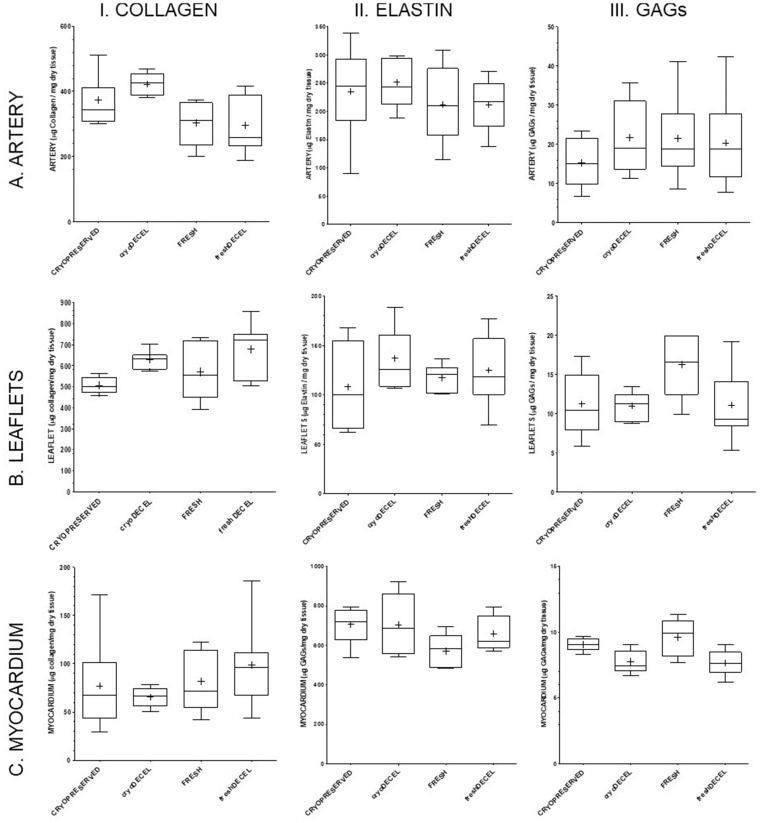
After decellularization, all tissues were evaluated macroscopically to ensure its good quality, obtaining a tissue with clean white artery and slightly brown myocardium (Figure 1a). With the intention of reducing the risk of structure damage provoked by the used reagents, main ECM biomolecules39 were quantified for the PV wall, leaflet and myocardium (Figure 3) to assure their preservation after the decellularization process. No significant differences after decellularization for collagen, the majority structural protein, neither elastin nor GAGs were observed. Decellularized samples were compared to both fresh and cryopreserved samples presenting a clear variability between donors.
Figure 3.
ECM biomolecules quantification. (AI) collagen pulmonary artery, (AII) elastin pulmonary artery, (AIII) GAGs pulmonary artery, (BI) collagen leaflets, (BII) elastin leaflets, (BIII) GAGs leaflets, (CI) collagen myocardium, (CII) elastin myocardium and (CIII) GAGs myocardium. Each graphic includes the results for native tissue (fresh or cryopreserved) and decellularized tissue (from fresh or cryopreserved). For the statistical analysis a non-parametric test Mann-Whitney double-tail has been performed with a minimum of N=5 and n=2. Significance level is p<0.05.
The structure was also evaluated in terms of biomechanical properties (Figure 4). The values obtained for maximum load, Young’s modulus, deformation at maximum load and stiffness of the analyzed tissue remain constant without significant differences between dPV and the native tissues. Thus, the intrinsic mechanical properties of the pulmonary artery are maintained after decellularization treatment. It is important to notice that there is a variability between donors. However, all the results obtained are within the same range, for both longitudinal and transversal measures, which implies that there are no significant differences between the analyzed groups (fresh PV vs dPV from fresh valve and cryopreserved PV and dPV from cryopreserved valve). In all cases, the mechanical properties analyzed are maintained in the same range, comparing with cryopreserved tissue, the actual gold standard.
Figure 4.
Biomechanical properties, obtained with uniaxial tensile test. For each heart valve group (N=5 each group) samples of two directions (longitudinal and transversal) were evaluated. (a) Comparison of different PV conditions in terms of Maximum load, Young Modulus, Elongation at rupture and Stiffness. (b) Strain-stress curve for longitudinal samples and (c) Stress-strain curves for transversal samples. Values are given as mean with standard deviation. PV, pulmonary heart valve; dPV, decellularized heart valve. For the statistical analysis a non-parametric test Mann-Whitney double-tail has been performed with a minimum of N=5. Significance level is considered for p<0.05.
In terms of hydrodynamic analyses, we performed i) a competence test to measure the leakage rate of the valves under physiological pressures (Figure 5) and ii) a pulsed flow test to evaluate the hydrodynamic behavior of the valve ring. A pressure range of 10–60 mmHg was analyzed. The PVs analyzed had good closure performance at several pressures, being moderately functional, although it varies depending on the donor: the average leakage flow at 10mmHg was 21.8 mL/min for the cryopreserved native valves and 36.2 mL/min for the decellularized ones. At higher pressures (60 mmHg) the leakage flow was 73.73 mL/min for the cryopreserved native valves and 126.76 mL/min for the decellularized ones. The difference between the two blocks does not refer to the protocol, but to the valves per se. With the analyzed results, no differences were observed between blocks, since the values obtained are in the same range. Among the 6 PV analyzed (3 cryopreserved and 3 cryopreserved decellularized), one of decellularized valves has an initial leakage flow at 10mmHg three times higher than the leakage flow of the cryopreserved native valves, while the other two valves of this block are in the same range. In addition, one of the cryopreserved native valves has a leakage flow an order of magnitude below the rest of the tissues. It would be necessary to increase the number of valves analyzed to address the variability associated with the donor.
Figure 5.
Competence test under static pressure of cryopreserved and cryopreserved decellularized pulmonary valves at a pressure range of 10–60mmHg. Continuous line (˗˗˗˗) states for cryopreserved tissues. Dotted line (···) states for decellularized tissues from cryopreserved samples.
On the other hand, the results of the functional test, demonstrate an excellent aperture in dynamic mode without apparent head loss between the entrance and the exit in all the analyzed valves. In average, the pressure loss between the entrance and the exit of the analysed valves was 3.96 ± 2.84 mmHg, with an opening time of 374 ± 651 ms at 60bpm. These values were 4.51 ± 6.84 mmHg & 183 ± 318 ms at 90 bpm and 3.21 ± 2.32 mmHg & 72 ± 59 ms at 120bpm. These results are in agreement with the founds in the literature.40 A functional example can be found in the Supplemental Figure 1.
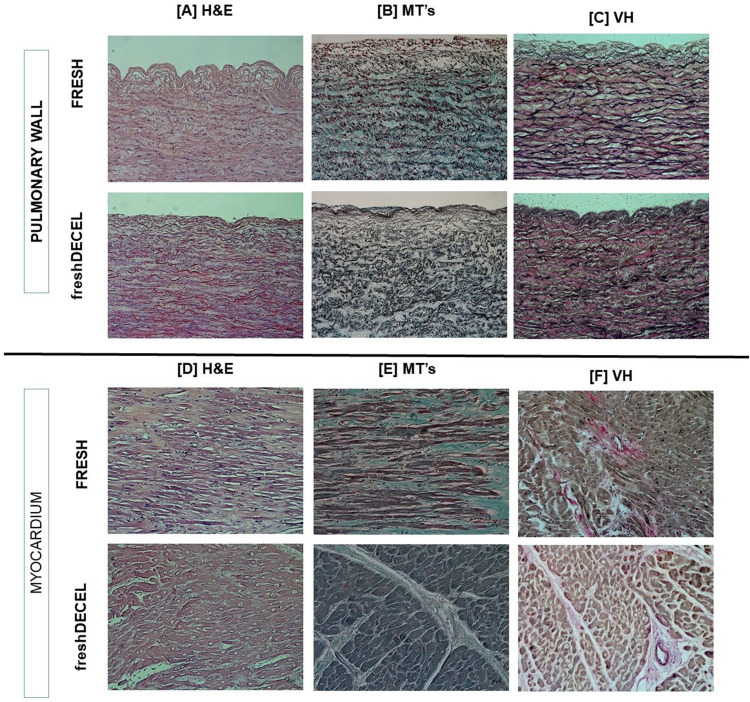
Additionally, histological samples were prepared to stain the tissues with three different staining’s (Figure 6). Specifically, pulmonary artery and myocardium were analyzed. Hematoxylin-Eosin preparations present dark blue cell nuclei staining for the native tissues, but it does not appear for the dPV. Masson trichromic shows that collagenous structure is maintained after processing. Moreover, elastic structure is maintained after decellularization process as evidenced by Verhoeff-Van Gieson staining. A complete panel of histology can be found in the Supplemental Figure 2.
Figure 6.
Tissue structure evaluation by histology (20x). Sections of arterial pulmonary wall and myocardium of 2 different conditions (fresh and fresh-decellularized tissue) stained with Haematoxylins-Eosin (H&E), Masson Trichrome (MS’s) and Verhoeff-Van Gieson (VH) staining. [A]Staining of pulmonary wall from two distinct conditions using H&E; [B] Staining of pulmonary wall from two distinct conditions using MT’s; [C] Staining of pulmonary wall from two distinct conditions using VH; [D] Staining of myocardium from two distinct conditions using H&E; [E] Staining of myocardium from two distinct conditions using; [F] Staining of myocardium from four distinct conditions using VH. A complete panel of tissue structure evaluation by histology of the four reported conditions (cryopreserved, cryopreserved-decellularized, fresh and fresh-decellularized) are included in supplementary information.
Evaluation of Cell Content After Decellularization: DNA Quantification
With the aim to reduce the risk related to the cellular content removal, DNA was quantified after decellularization for all tissues comprising the homografts accomplishing DNA content below 50 ng DNA/mg dry tissue (Figure 7). The amount of native tissue DNA varies between 1.000 and 10.000 ng/mg dry tissue, depending on the tissue (artery, myocardium or leaflet), its origin (fresh or cryopreserved) and the donor. Tissues which were cryopreserved previous decellularization present the lowest DNA quantity. Moreover, myocardium tissue is harder to decellularize, maintaining a quantity around 19,65 ± 13,43 ng/mg dry tissue after decellularization. In any case, the proposed protocol accomplishes a 99% of genetic material elimination. Agarose gel (data not shown) confirmed the absence of remaining fragments bigger than 200 bp.
Figure 7.
DNA quantity given in ng/mg dry tissue after decellularization of fresh pulmonary heart valves (fresh, N=5-7; freshDECEL, N = 6–9) and cryopreserved pulmonary heart valves (cryopreserved, N=5; *DECEL, N = 5–7). DNA was quantified by fluorescence detection using PicoGreen™ dye.
Evaluation of the Microbiology During the Process
There was no evidence of bacterial or fungal growth on either the thioglycolate media or the blood culture for any of the samples taken during the process. Thus, the designed processing is microbiologically safe since all quality controls that monitor in the microbiology of the process were negative.
Evaluation of Final Product Cytotoxicity
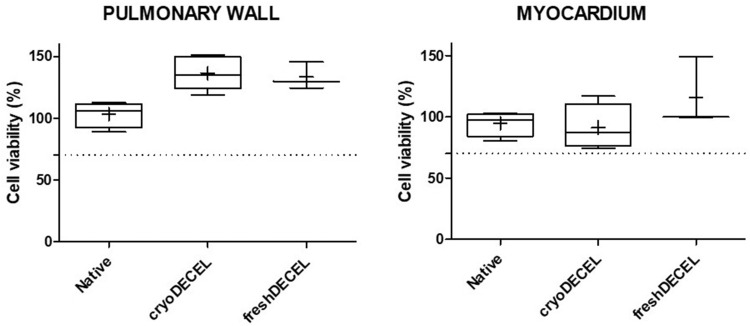
The cytotoxicity assay to assess the cytotoxic potential of remaining components from the decellularization process and the decellularized matrix was performed using the extracts of pulmonary wall and myocardium of fresh, cryopreserved and fresh decellularized heart valve tissue. The results of the quantitative analyses for the cell viability are in line with the definition of cell toxicity given by ISO 10993–5, considering a reduction of cell viability by more than 30% as cytotoxic. As shown in Figure 8, the mean cellular viability for all tissue processing groups is above 70%, indicating that the decellularized matrix has no cytotoxic effects.
Figure 8.
Cytotoxic assay. Cell viability of 3T3 cells (murine fibroblast) incubated for 24 hours with tissue extract prepared from fresh (N=4), decellularized (N=5), and fresh decellularized (BTB, N=3) pulmonary heart valve tissue. Cell viability is expressed as percentage relative to the negative control (culture medium) as 100% of viability. Positive cytotoxic control prepared with 0.1% Triton X-100, V/V) results to 0.8% of viability. Experiments were performed as technical triplicates using a density of 10.000 cells per well.
Final Risk Assessment
After analysing the results of the proposed in vitro studies, a re-evaluation using EuroGTP II IAT was conducted to assess the extent of risk mitigation (see Table 2). As described in previous section, four studies were proposed to mitigate the risks originally identified: (i) evaluation of integrity of tissue structure; (ii) evaluation of cell content, (iii) evaluation of the microbiology safety and (iv) evaluation of cytotoxicity in the final tissue preparation. The rationale and scoring obtained for each risk factor considering the results obtained are recorded in Table 2, which also includes the percentage of risk reduction to adjust the score based on external data (comprising published literature and unpublished data from external sources). In terms of cellular content removal, it was demonstrated a 99% removal of DNA content. Despite the perceived low risk, since the cryopreserved heart valves being implanted have historically contained cellular contents without eliciting significant reactions, reducing the DNA below the threshold of 50 ng/mg dry tissue, according to previous publications, decreases the probability from possible to rare. Additionally, protein quantification, mechanical analysis, and histology revealed no tissue damage, further decreasing the likelihood of implant failure from possible to unlikely. Regarding disease transmission, a microbiological quality control process has been implemented, enabling confirmation of the absence of contamination and enhancing the ability to detect it promptly if it occurs prior to issuing tissues for transplant. These findings have significantly enhanced our ability to detect any potential contamination to a very high level. Furthermore, the in vitro results have shown no cytotoxicity, thereby reducing the probability of graft toxicity in vivo to rare levels.
Table 2.
Reevaluation of the Risk Linked to the Implementation and Clinical Application of dPV Following in vitro Testing
| Risk factors | Does it apply? | Justification | Risk consequences | P | S | D | PR | RR (%) | Risk score |
|
|---|---|---|---|---|---|---|---|---|---|---|
| Preparation process | Processing and environment | Yes | After decellularization DNA quantity is below the threshold (50ng/mg dry tissue), decreasing the possibility of unwanted immunogenicity | Unwanted immunogenicity | 1 | 1 | 5 | 5 | 9514,19,41–46 | 0.25 |
| After validation the risk of contaminating the grafts during processing has not been observed. Moreover, several microbiological controls have been added during processing which allow to confirm the absence of contamination, and increase the detection it in case it occurs before issuing tissues for transplant. | Disease transmission |
2 | 3 | 1 | 6 | 5014, FVTB internal validation | 3 | |||
| Reagents | Yes | The decellularization process requires several additional reagents, which have been priory used in our TE for other procedures. The results obtained after in vitro evaluation do not present any damage of the tissue structure. | Implant Failure | 2 | 4 | 4 | 32 | 2514,43,47–49 | 24 | |
| Adding different solutions during the preparation process, may increase the risk of tissue contamination during processing, comparing with the current processing protocol. However, the used reagents are sterile or are filtered before their use and several microbiological controls have been included during processing | Disease transmission |
1 | 2 | 1 | 2 | 2550–52 | 1.5 | |||
| After validation, we have not observed any cytotoxicity in the vitro studies performed. | Toxicity / carcinogenicity | 1 | 2 | 5 | 10 | 9543,44,46,53,54 | 0.5 | |||
| Reliability of microbiology testing | Yes | The reagents used may affect the reliability of testing in different stages of processing. NaCl and enzymatic treatment does not seem to affect our ability to detect contamination. The use of antibiotics and their impact on the reliability of testing have been validated for other processes, and does not represent a risk. | Disease transmission |
1 | 2 | 1 | 2 | 25 (external test reliability validation has been performed for the decellularization reagents) | 1.5 | |
| Storage conditions | Yes | The storage method may damage the structure of the decellularized tissue. | Implant Failure | 3 | 4 | 3 | 36 | 0 | 36 | |
| Preliminary Score (Σ individual risk scores) | 66.25 | |||||||||
| Combined Risk Value ((Preliminary Score x Highest Possible Score)/((Max S × Max P × Max D) × Number of applicable risk consequences) = (66.25 x 4500)/(100 x 7) | 425.89 | |||||||||
| Final Risk Score ((Combined Risk Value ×100)/ Highest Possible Score) | 10 | |||||||||
Notes: Adapted from the EuroGTP-II guidelines (EuroGTP-II, 2019).36 Green: Low risk level, Orange: Moderate risk level, Red: high risk level.
Abbreviations: D, detectability; P, probability; PR, potential risk; RR, risk reduction; S, severity.
Discussion
Current options for pulmonary valve replacement include homografts, xenograft-based valves, and mechanical valves. Mechanical valves require lifelong anticoagulant therapy, which limits their use in younger patients and women of childbearing age. Xenografts are potentially more accessible and cost-effective alternative; however, their use is limited by the risk of acute immune responses. These responses often lead to accelerated calcification, significantly shortening the functional lifespan of the valve.55,56 Homografts, on the other hand, demonstrate superior performance in terms of durability and resistance, making them less susceptible to infections. Their low immunogenicity significantly reduces the risk of calcification, contributing to greater longevity.57 However, their primary limitation lies in their restricted availability, as the supply of donated human grafts remains nowadays insufficient to meet clinical demand. Nowadays, the use of decellularized cardiac valve allografts in clinical practice is on the rise, but their high cost and limited accessibility makes them unaffordable for some public health systems and inaccessible for patients. Nowadays, different options of dPV can be found commercially such as CryoValve® SynerGraft (SG) from Cryolife® which uses SyneGraft technology and has received the premarket notification from the US Food and Drug Administration,45,58 CardioPure HV from Tissue Regenix using dCell technology59–61 and Espoir PV from Corlife.62,63 In this context, as TE, we consider it crucial to be capable of addressing this clinical requirement in order to ensure accessibility of this product to the wider population. During the last years, EuroGTPII methodologies have highlighted the importance of anticipating associated risks related with changes applied to specific processes.31,33,34,36,64 This work pinpoints the risks associated with the clinical application of a newly developed process for the decellularization of PV grafts for valve replacement, enabling us to evaluate the consequences and overall risk level. The resulting initial risk assessment yielded a Final Risk Score of 23, indicating a high-risk level, and dictating the necessary extent of studies for ensuring the safety and efficacy of dVP grafts through pre-clinical and clinical evaluations.
The initial strategy for mitigating the risk associated with the newly developed procedure involves gathering additional evidence to support the safe and effective utilisation of the new product, followed by implementing risk mitigation measures. Within this framework, an in vitro validation was conducted to mitigate the risk associated with the process, consisting of pre-clinical in vitro evaluation studies tailored to tackle risks. Multiple in-vitro studies were proposed to target each specific risk encompassing unintended immunogenicity, implant failure, disease transmission, and toxicity/carcinogenicity. The forthcoming studies will involve pre-clinical in vivo evaluation using animal models.
The primary goal of decellularization is to remove cellular components, protecting ECM structure from the direct and semi-direct pathways, limiting allorecognition to the indirect pathway, which is less prone to trigger acute rejection. Consequently, decellularized ECM scaffolds are noted for their higher biocompatibility and lower rejection rate compared to alternative implants.65 Through the proposed in vitro analysis, it can be concluded that the proposed process effectively eliminates 99% of DNA across all analysed tissues (pulmonary wall, myocardium, and leaflets), with the remaining DNA consistently below the threshold (50ng/mg dry tissue). These findings align with prior publications utilising various decellularization protocols for human valve decellularization. For instance, Vafaee et al reported more than 97% of DNA elimination (<20 ng/mg) using a 10 days protocol combining hypotonic solutions, detergents and nuclease digestion for cryopreserved human valve decellularization,43 while Iop et al reported a negligible residual DNA content (<7 ng/mg) with an average loss of 99.5% for human aortic valves using TRICOL protocol.14 The TRICOL protocol includes Triton X-100 as a decellularization agent. However, due to its toxic effects on aquatic organisms, it has been added to the European Authorization list of REACH. Consequently, since 2021, its use is prohibited unless authorized by the authorities or exempt from authorization.66,67 Additional studies have assessed the reduction of DNA in heart valves of animal origin, consistently observing a significant decrease in DNA content across all analysed tissues.19,44,46 Other studies, demonstrate cellularity removal through other techniques such as microscopic examination45 or PCR.42 In light of these studies, the proposed protocol offers several advantages, including cost-effectiveness and enhanced scalability, attributed to the shorter procedure duration and the absence of detergents, which also addresses environmental concerns.
Importantly, as balance of DNA elimination it is paramount to maintain the ECM structure. Although since 2022 till now, multiple publications have appeared regarding decellularized heart valves implantation in humans, most of them showing promising results,47–49 there is no extensive in vitro bibliography regarding decellularization protocol’s effect on ECM structure. Implant failure was identified as a consequence of ECM alteration due to two risk factors: reagents and preservation. This study addresses the decellularization process itself rather than the preservation method, thereby concentrating solely on the assessment of reagents. Research indicating adverse alterations in ECM architecture commonly employed aggressive decellularization methods,58 while gentler protocols can strike a balance between removing cellular components and preserving ECM structure.
In order to reduce this risk, the integrity of the ECM has been confirmed through a series of in vitro tests, including histology, main ECM components quantification and mechanical assay. Histological evaluation through H&E, MT and VH staining’s confirmed the preservation of structure, consistent with findings from other protocols used on human valves as reported previously.14,43,46 Although prior research has indicated the impact of detergent reagents on the protein content,53 in this case protein quantification confirms the preservation of ECM, primarily made up of densely aligned collagen fibres that provide structural strength, elastin that allows for stretch and retraction during the cardiac cycle, and glycosaminoglycans (GAGs) that enable the relative movements between adjacent layers.39 For instance, Vafaee et al showed a reduction of GAGs,43 while other studies confirmed elastin and collagen integrity through two-photon laser scanning confocal microscopy within heart valve leaflets after decellularization through SynerGraft procedure.58
Mechanical behaviour of the obtained dPV was assessed to mitigate the risk of implant failure. Comparison between the dPV and native tissue indicates that the tissue’s inherent mechanical properties remain within the same range post-decellularization. This data contrasted with previous published protocols, where moderate changes in the maximum load to failure of the tissues were recorded post decellularization.43 On the other hand, results from hydrodynamic assay suggest that the analysed valves exhibit satisfactory performance under static pressure and maintain excellent aperture in dynamic mode without noticeable loss of load from entry to exit, as previously published by Desai et. al for dPV and aortic roots using low concentration of SDS.68 CryoValve® SG demonstrated equivalent hydrodynamic performance with mean systolic pressure gradients less than 5 mmHg at all flow conditions.45 Additionally, the mechanical behaviour analysis of acellular porcine pulmonary valve scaffolds showed minimal impact from various decellularization protocols.69,70
Typically, decellularization agents are employed for their innate properties to disrupt cell membranes and eliminate cellular content. However, if these agents persist within the tissue at elevated concentrations post-treatment, they may pose toxicity to host cells, hindering cellular infiltration during recellularization processes, both in vitro and in vivo.53 For example, detergents such as TX100 or SDS which are commonly used in some decellularization protocols, have been related with a certain grade of cytotoxicity. Bearing this concern in mind, we have systematically avoided theses detergents from our experimental protocol.65 In this instance, the post-decellularization final washes applied to ensure the production of biocompatible tissue, consistent with previously published protocols for decellularizing human valves.43 Moreover, multiple decellularization protocols applied for different tissues have demonstrated the obtaining of biocompatible tissue.38,71–74
Regarding disease transmission, three different risk factors were identified: processing and environment, reagents and reliability of microbiology testing, so that various perspectives have been considered to assess the associated risk. It appears evident that an increased number of process steps may elevate the risk of contamination. Nevertheless, the approach outlined in this study commences with a decontamination step utilizing antibiotics, and the decellularization protocol itself, conducted under aseptic conditions, acts as an indirect decontamination process.50–52 Following validation in BST facilities, there have been no observed instances of graft contamination during processing, a trend similarly noted in the Fondazione Banca dei Tessuti del Veneto (FBTV) facilities (internal validation, data not shown). Additionally, consistent with previous studies,14 several microbiological controls have been incorporated throughout the process to verify the absence of contamination and enhance detection in the event of occurrence before realising tissues for transplant. In terms of reliability, internal validation was conducted to ensure that any potential microbial growth could be detected using the specified method, thus mitigating associated risks.
Based on the results obtained from the in vitro studies and the available data, reassessment using the algorithm in the IAT indicated a moderate level of risk (Final Risk Score = 10), suggesting the need for additional evidence to support the safe and effective clinical application of this new tissue preparation. Considering the completion of feasible in vitro studies within the TE, the proposal is to proceed with further pre-clinical in vivo evaluation to address and mitigate the remaining specific risks identified, utilizing an animal model. Furthermore, the outstanding risk associated with implant failure related to tissue storage will be tackled through additional in vitro studies aimed at demonstrating structural maintenance, thus reducing the risk score. Those studies will provide data on tissue stability to help determine the best procedure for tissue packaging and storage, with the aim of having decellularized valves readily available, aiming to achieve or maintain a longer shelf life than currently available.
Conclusion
The current investigation addresses the necessary steps for safely implanting a new decellularization protocol for PV into our TE’s regular procedures. These steps include assessing novelty, conducting risk evaluations, designing and executing studies to mitigate identified risks. EuroGTPII methodologies allow identifying and quantifying the risks associated with the introduction of innovation in TE activities and thereafter implement a set of analysis to mitigate the risk, thereby promoting transparency and expediting authorization procedures by competent authorities. The high level of risk determined for the novel dPV preparation process led to a set of in vitro studies that helped to mitigate the potential risk consequences and guaranty a safely implement tissue preparation into a clinical practice in a future. The in vitro studies proposed were capable to diminish the risk related with unwanted immunogenicity, disease transmission, implant failure and toxicity, while the external sources of information reduced the overall risk score. After this study, the risk has been reduced from high (23 risk score) to moderate risk (10 risk score). Therefore, the present work provides evidence that dPVs can be obtained using the proposed protocol, maintaining the specifications described for the cryopreserved pulmonary valves. This result is an affordable alternative for the health systems, and determines the feasibility of preparing these tissues within traditional tissue establishment facilities, turning this SoHO preparation a valuable therapeutic opportunity for EU patients in need.
Acknowledgments
We would like to thank to all donors and their relatives for the altruistic act of donation. We would also like to thank transplant coordinators, Donor Centre, recovery teams and technical processing team for their efforts that make it possible to obtain the tissues used in this study. This work has been partially supported by an internal competitive grant from BST (REF I.2018.27), and Red de Investigación Cooperativa Orientada a Resultados en Salud (RICORS) TERAV (RD21/0017/0002; RD21/0017/0022), from the Instituto de Salud Carlos III Madrid, Spain.
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Abbreviations
BST, Banc de Sang I Teixits – “Barcelona Tissue Bank”; CFUpP, Clinical Follow-up Plan; PV, Pulmonary valve; DNA, Deoxyribonucleic acid; ECM, Extracellular matrix; dPV, Decellularized Pulmonary valve; EDQM, European Directorate for the Quality of Medicines and Health Care; EuroGTP II, Good Practices for demonstrating safety and quality through recipient follow up; EUTCD, European Union Tissues and Cells Directives; GAG, glycosaminoglycan; ON, Over night; H&E, haematoxylin and eosin; IAT, Interactive Assessment Tool; MT, Masson’s Trichrome; PBS, Phosphate buffer saline; RECm, Research Ethics Committee for Medicinal Products; SoHO, Substances of Human Origin; TE, Tissue Establishment; VH, Verhoeff-Van Gieson.
Consent to Participate
All tissue samples come from donors whose family signed informed consent. Availability of data and material: no supporting data is available.
Ethical Statement
This study followed the ethical precepts of the Declaration of Helsinki and was approved by the local ethics committee. Human tissue was processed according to guidance for clinical use (EEC regulations 2004/23/CE and 2006/17/CE) and to the legal requirements for the use of biological samples for research in Spain (Law 14/2007 and RD 1716/2011). An ethics institutional review board (IRB) approval was obtained (CEIm Hospital Valle Hebrón, Barcelona; PR (BST) 315/2019).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Lisy M, Kalender G, Schenke-Layland K, Brockbank KGM, Biermann A, Stock UA. Allograft heart valves: current aspects and future applications. Biopreserv Biobank. 2017;15(2):148–157. doi: 10.1089/bio.2016.0070 [DOI] [PubMed] [Google Scholar]
- 2.Khanna SK, Ross JK, Monro JL. Homograft aortic valve replacement: seven years’ experience with antibiotic-treated valves. Thorax. 1981;36(5):330–337. doi: 10.1136/thx.36.5.330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spatenka J, Burkert J. Allograft heart valve in aortic valve surgery. Aortic Regurgitation. 2018;155–168. [Google Scholar]
- 4.O’Brien MF, Stafford EG, Gardner MAH, Pohlner PG, McGiffin DC, Kirklin JW. A comparison of aortic valve replacement with viable cryopreserved and fresh allograft valves, with a note on chromosomal studies. J Thorac Cardiovasc Surg. 1987;94(6):812–823. doi: 10.1016/s0022-5223(19)36152-5 [DOI] [PubMed] [Google Scholar]
- 5.Kostyunin AE, Yuzhalin AE, Rezvova MA, Ovcharenko EA, Glushkova TV, Kutikhin AG. Degeneration of bioprosthetic heart valves: update 2020. J Am Heart Assoc. 2020;9(19):1–19. doi: 10.1161/JAHA.120.018506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neishabouri A, Soltani Khaboushan A, Daghigh F, Kajbafzadeh AM, Majidi Zolbin M. Decellularization in tissue engineering and regenerative medicine: evaluation, modification, and application methods. Front Bioeng Biotechnol. 2022;10(April):1–21. doi: 10.3389/fbioe.2022.805299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crapo PM, Gilbert TW, Badylak SF. An Overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32(12):3233–3243. doi: 10.1016/j.biomaterials.2011.01.057.An [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oripov F, Ramm R, Falk C, et al. Serial assessment of early antibody binding to decellularized valved allografts. Front Cardiovasc Med. 2022;9(August):1–11. doi: 10.3389/fcvm.2022.895943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Helder MRK, Kouchoukos NT, Zehr K, et al. Late durability of decellularized allografts for aortic valve replacement: a word of caution. J Thorac Cardiovasc Surg. 2016;152(4):1197–1199. doi: 10.1016/j.jtcvs.2016.03.050 [DOI] [PubMed] [Google Scholar]
- 10.Baraki H, Tudorache I, Braun M, et al. Orthotopic replacement of the aortic valve with decellularized allograft in a sheep model. Biomaterials. 2009;30(31):6240–6246. doi: 10.1016/j.biomaterials.2009.07.068 [DOI] [PubMed] [Google Scholar]
- 11.Gallo M, Naso F, Poser H, et al. Physiological performance of a detergent decellularized heart valve implanted for 15 months in Vietnamese pigs: surgical procedure, follow-up, and explant inspection. Artif Organs. 2012;36(6):138–150. doi: 10.1111/j.1525-1594.2012.01447.x [DOI] [PubMed] [Google Scholar]
- 12.Sarikouch S, Horke A, Tudorache I, et al. Decellularized fresh homografts for pulmonary valve replacement: a decade of clinical experience. Eur J Cardiothorac Surg. 2016;50(2):281–290. doi: 10.1093/ejcts/ezw050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neumann A, Cebotari S, Tudorache I, Haverich A, Sarikouch S. Heart valve engineering: decellularized allograft matrices in clinical practice. Biomed Tech. 2013;58(5):453–456. doi: 10.1515/bmt-2012-0115 [DOI] [PubMed] [Google Scholar]
- 14.Iop L, Paolin A, Aguiari P, Trojan D, Cogliati E, Gerosa G. Decellularized cryopreserved allografts as off-The-shelf allogeneic alternative for heart valve replacement in vitro assessment before clinical translation. J Cardiovasc Translat Res. 2017;10(2):93–103::1–6. doi: 10.1007/s12265-017-9738-0 [DOI] [PubMed] [Google Scholar]
- 15.Badria AF, Koutsoukos PG, Mavrilas D. Decellularized tissue-engineered heart valves calcification: what do animal and clinical studies tell us?. J Mater Sci Mater Med. 2020;31(12). doi: 10.1007/s10856-020-06462-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huyan Y, Chang Y, Song J. Application of homograft valved conduit in cardiac surgery. Front Cardiovasc Med. 2021;8(October):1–15. doi: 10.3389/fcvm.2021.740871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hopkins RA, Jones AL, Wolfinbarger L, Moore MA, Bert AA, Lofland GK. Decellularization reduces calcification while improving both durability and 1-year functional results of pulmonary homograft valves in juvenile sheep. J Thorac Cardiovasc Surg. 2009;137(4):907–913.e4. doi: 10.1016/j.jtcvs.2008.12.009 [DOI] [PubMed] [Google Scholar]
- 18.Lehr EJ, Rayat GR, Chiu B, et al. Decellularization reduces immunogenicity of sheep pulmonary artery vascular patches. J Thorac Cardiovasc Surg. 2011;141(4):1056–1062. doi: 10.1016/j.jtcvs.2010.02.060 [DOI] [PubMed] [Google Scholar]
- 19.Wan J, Zhong X, Xu Z, et al. A decellularized porcine pulmonary valved conduit embedded with gelatin. Artif Organs. 2021;45(9):1068–1082. doi: 10.1111/aor.13955 [DOI] [PubMed] [Google Scholar]
- 20.Cebotari S, Lichtenberg A, Tudorache I, et al. Clinical application of tissue engineered human heart valves using autologous progenitor cells. Circulation. 2006;114(SUPPL. 1). doi: 10.1161/CIRCULATIONAHA.105.001065 [DOI] [PubMed] [Google Scholar]
- 21.Cebotari S, Tudorache I, Ciubotaru A, et al. Use of fresh decellularized allografts for pulmonary valve replacement may reduce the reoperation rate in children and young adults: early report. Circulation. 2011;124(11 SUPPL. 1). doi: 10.1161/CIRCULATIONAHA.110.012161 [DOI] [PubMed] [Google Scholar]
- 22.Bobylev D, Breymann T, Boethig D, Haverich A, Ono M. Semilunar valve replacement with decellularized homograft after Damus-Kaye-Stansel anastomosis and Fontan procedure. Ann Thorac Surg. 2014;97(5):1792–1795. doi: 10.1016/j.athoracsur.2013.07.116 [DOI] [PubMed] [Google Scholar]
- 23.EDQM; Council of Europe. Guide to the Quality and Safety of Tissues and Cells for Human Application. 5th Edition. 4th ed. 2022. [Google Scholar]
- 24.de By TMMH, Parker R, Delmo Walter EM, Hetzer R. Cardiovascular tissue banking in Europe. HSR Proc Intensive Care Cardiovasc Anesth. 2012;4(4):251–260. [PMC free article] [PubMed] [Google Scholar]
- 25.Ruzmetov M, Shah JJ, Geiss DM, Fortuna RS. Decellularized versus standard cryopreserved valve allografts for right ventricular outflow tract reconstruction: a single-institution comparison. J Thorac Cardiovasc Surg. 2012;143(3):543–549. doi: 10.1016/j.jtcvs.2011.12.032 [DOI] [PubMed] [Google Scholar]
- 26.Gallo M, Bonetti A, Poser H, et al. Decellularized aortic conduits: could their cryopreservation affect post-implantation outcomes? A morpho-functional study on porcine homografts. Heart Vessels. 2016;31(11):1862–1873. doi: 10.1007/s00380-016-0839-5 [DOI] [PubMed] [Google Scholar]
- 27.Brown JW, Elkins RC, Clarke DR, et al. Performance of the CryoValve* SG human decellularized pulmonary valve in 342 patients relative to the conventional CryoValve at a mean follow-up of four years. J Thorac Cardiovasc Surg. 2010;139(2):339–348. doi: 10.1016/j.jtcvs.2009.04.065 [DOI] [PubMed] [Google Scholar]
- 28.Simon P, Kasimir MT, Seebacher G, et al. Early failure of the tissue engineered porcine heart valve SYNERGRAFT® in pediatric patients. Eur J Cardiothorac Surg. 2003;23(6):1002–1006. doi: 10.1016/S1010-7940(03)00094-0 [DOI] [PubMed] [Google Scholar]
- 29.Petrisli E, Carella C, Navarro A, Fehily D, Strong D, Cardillo M. Vigilance for medical products of human origin - progress on the notify library’s global effort to share information and learning. Transplantation. 2021;105(9):1921–1929. doi: 10.1097/TP.0000000000003589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.López-Chicón P, Pérez M, Castells-Sala C, et al. Quality by design: development of safe and efficacious full-thickness acellular dermal matrix based on EuroGTPII methodologies. Manuscr Submitt Publ. 2022; 567–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trias E, Lomas R, Tabera J, et al. EuroGTP II: a tool to assess risk, safety and efficacy of substances of human origin. Int J Qual Heal Care. 2019;32(1):80–84. doi: 10.1093/intqhc/mzz048 [DOI] [PubMed] [Google Scholar]
- 32.EuroGTP (2007 207) - Good Tissue Practices. No Title. Available from: http://eurogtps.com. Accessed May 2, 2023.
- 33.Trias E, Gallon P, Ferrari S, et al. Banking of corneal stromal lenticules: a risk-analysis assessment with the EuroGTP II interactive tool. Cell Tissue Bank. 2019;21(2):189–204. doi: 10.1007/s10561-020-09813-8 [DOI] [PubMed] [Google Scholar]
- 34.Trias E, Nijs M, Rugescu IA, et al. Evaluating risk, safety and efficacy of novel reproductive techniques and therapies through the EuroGTP II risk assessment tool. Hum Reprod. 2020;35(8):1821–1838. doi: 10.1093/humrep/deaa146 [DOI] [PubMed] [Google Scholar]
- 35.Lierman S, Bus A, Andries S, Trias E, Bols PEJ, Tilleman K. Passive slow freezing is an efficacious and cost-effective alternative to controlled slow freezing for ovarian tissue cryopreservation. Cryobiology. 2021;100(December 2020):164–172. doi: 10.1016/j.cryobiol.2021.01.013 [DOI] [PubMed] [Google Scholar]
- 36.EuroGTPII (709567)-. Good Practices for Demonstrating Safety and Quality Through Recipient Follow-Up. Euro GTP II Guide - Good Practices for Evaluating Safety, Quality and Efficacy of Tissue and Cellular Therapies and Products. [Google Scholar]
- 37.Castells-Sala C, Pérez ML, Agustí E, et al.. Last twenty-years activity of cardiovascular tissue banking in Barcelona. Cell Tissue Bank. 2023;0123456789. doi: 10.1007/s10561-022-10059-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pérez ML, Castells-Sala C, López-Chicón P, et al. Fast protocol for the processing of split-thickness skin into decellularized human dermal matrix. Tissue Cell. 2021;72:101572. doi: 10.1016/j.tice.2021.101572 [DOI] [PubMed] [Google Scholar]
- 39.Kodigepalli KM, Thatcher K, West T, et al. Biology and biomechanics of the heart valve extracellular matrix. J Cardiovasc Dev Dis. 2020;7(4):1–22. doi: 10.3390/jcdd7040057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bakhtiary F, Dzemali O, Steinseiffer U, et al. Opening and closing kinematics of fresh and calcified aortic valve prostheses: an in vitro study. J Thorac Cardiovasc Surg. 2007;134(3):657–662. doi: 10.1016/j.jtcvs.2007.02.050 [DOI] [PubMed] [Google Scholar]
- 41.van Steenberghe M, Schubert T, Gerelli S, et al. Porcine pulmonary valve decellularization with NaOH-based vs detergent process: preliminary in vitro and in vivo assessments. J Cardiothorac Surg. 2018;13(1):34. doi: 10.1186/s13019-018-0720-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Godehardt AW, Ramm R, Gulich B, Tönjes RR, Hilfiker A. Decellularized pig pulmonary heart valves—Depletion of nucleic acids measured by proviral PERV pol. Xenotransplantation. 2020;27(2):1–11. doi: 10.1111/xen.12565 [DOI] [PubMed] [Google Scholar]
- 43.Vafaee T, Thomas D, Desai A, et al. Decellularization of human donor aortic and pulmonary valved conduits using low concentration sodium dodecyl sulfate. J Tissue Eng Regen Med. 2018;12(2):e841–e853. doi: 10.1002/term.2391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.İnal MS, Darcan C, Akpek A. Characterization of a decellularized sheep pulmonary heart valves and analysis of their capability as a xenograft initial matrix material in heart valve tissue engineering. Bioengineering. 2023;10(8):949. doi: 10.3390/bioengineering10080949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elkins RC, Dawson PE, Goldstein S, Walsh SP, Black KS. Decellularized human valve allografts. Ann Thorac Surg. 2001;71(5):S428–S432. doi: 10.1016/S0003-4975(01)02503-6 [DOI] [PubMed] [Google Scholar]
- 46.Theodoridis K, Müller J, Ramm R, et al. Effects of combined cryopreservation and decellularization on the biomechanical, structural and biochemical properties of porcine pulmonary heart valves. Acta Biomater. 2016;43:71–77. doi: 10.1016/j.actbio.2016.07.013 [DOI] [PubMed] [Google Scholar]
- 47.Horke A, Tudorache I, Laufer G, et al. Early results from a prospective, single-arm European trial on decellularized allografts for aortic valve replacement: the ARISE study and ARISE Registry data. Eur J Cardiothorac Surg. 2020;58(5):1045–1053. doi: 10.1093/ejcts/ezaa100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bobylev D, Horke A, Boethig D, et al. 5-Year results from the prospective European multi-centre study on decellularized homografts for pulmonary valve replacement ESPOIR Trial and ESPOIR Registry data. Eur J Cardiothorac Surg. 2022;62(5):ezac219. doi: 10.1093/ejcts/ezac219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Söylen B, Sarikouch S, Horke A. Research & reviews : journal of nursing & health sciences improved results using decellularized human valves for children with congenital heart defects research & reviews. J Nurs Health Sci. 2016;3(1):7–10. [Google Scholar]
- 50.Michon AL, Jumas-Bilak E, Chiron R, Lamy B, Marchandin H. Advances toward the elucidation of hypertonic saline effects on pseudomonas aeruginosa from cystic fibrosis patients. PLoS One. 2014;9(2):1–8. doi: 10.1371/journal.pone.0090164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perera KC, Ekanayaka SK, Chandrasiri NS, Jayatilleke K, Kottahachchi J. In-vitro evaluation of bactericidal activity of antiseptics and disinfectants commonly used in healthcare settings. Gall Med J. 2021;26(1):23–30. doi: 10.4038/gmj.v26i1.8079 [DOI] [Google Scholar]
- 52.Tetz GV, Artemenko NK, Tetz VV. Effect of DNase and antibiotics on biofilm characteristics. Antimicrob Agents Chemother. 2009;53(3):1204–1209. doi: 10.1128/AAC.00471-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hussein KH, Park KM, Kang KS, Woo HM. Biocompatibility evaluation of tissue-engineered decellularized scaffolds for biomedical application. Vol 67 Elsevier B V. 2016. doi: 10.1016/j.msec.2016.05.068 [DOI] [PubMed] [Google Scholar]
- 54.Luo J, Korossis SA, Wilshaw SP, Jennings LM, Fisher J, Ingham E. Development and characterization of acellular porcine pulmonary valve scaffolds for tissue engineering. Tissue Eng - Part A. 2014;20(21–22):2963–2974. doi: 10.1089/ten.tea.2013.0573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mokryk I, Schmitz C. Natural biomaterials in the management of the aortic valve pathology. Biomed Clin Aspects. 2024;1–8. doi: 10.17305/bb.2024.11009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Emani SM. Options for prosthetic pulmonary valve replacement. Semin Thorac Cardiovasc Surg Pediatr Card Surg Ann. 2012;15(1):34–37. doi: 10.1053/j.pcsu.2012.01.015 [DOI] [PubMed] [Google Scholar]
- 57.Galeone A, Trojan D, Gardellini J, Di Gaetano R, Faggian G, Luciani GB. Cryopreserved aortic homografts for complex aortic valve or root endocarditis: a 28-year experience. Eur J Cardiothorac Surg. 2022;62(3):1–9. doi: 10.1093/ejcts/ezac193 [DOI] [PubMed] [Google Scholar]
- 58.Gerson CJ, Elkins RC, Goldstein S, Heacox AE. Structural integrity of collagen and elastin in SynerGraft® decellularized-cryopreserved human heart valves. Cryobiology. 2012;64(1):33–42. doi: 10.1016/j.cryobiol.2011.11.001 [DOI] [PubMed] [Google Scholar]
- 59.Da Costa FDA, Costa ACBA, Prestes R, et al. The early and midterm function of decellularized aortic valve allografts. Ann Thorac Surg. 2010;90(6):1854–1860. doi: 10.1016/j.athoracsur.2010.08.022 [DOI] [PubMed] [Google Scholar]
- 60.da Costa FDA, Takkenberg JJM, Fornazari D, et al. Long-term results of the Ross operation: an 18-year single institutional experience. Eur J Cardiothorac Surg. 2014;46(3):415–422. doi: 10.1093/ejcts/ezu013 [DOI] [PubMed] [Google Scholar]
- 61.Booth C, Korossis SA, Wilcox HE, et al. Tissue engineering of cardiac valve prostheses I: development and histological characterization of an acellular porcine scaffold. J Heart Valve Dis. 2002;11(4):457–462. [PubMed] [Google Scholar]
- 62.Boethig D, Horke A, Hazekamp M, et al. A European study on decellularized homografts for pulmonary valve replacement: initial results from the prospective ESPOIR Trial and ESPOIR Registry data. Eur J Cardiothorac Surg. 2019;56(3):503–509. doi: 10.1093/ejcts/ezz054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jashari R. Transplantation of cryopreserved human heart valves in Europe: 30 years of banking in Brussels and future perspectives. Cell Tissue Bank. 2021;22(4):519–537. doi: 10.1007/s10561-021-09902-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.López-Chicón P, Pérez ML, Castells-Sala C, et al. Quality by design: development of safe and efficacious full-thickness acellular dermal matrix based on EuroGTPII methodologies. Ther Clin Risk Manag. 2023;19(June):567–578. doi: 10.2147/TCRM.S410574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kasravi M, Ahmadi A, Babajani A, et al. Immunogenicity of decellularized extracellular matrix scaffolds: a bottleneck in tissue engineering and regenerative medicine. Biomater Res. 2023;27(1):1–24. doi: 10.1186/s40824-023-00348-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jho EH, Yun SH, Thapa P, Nam JW. Changes in the aquatic ecotoxicological effects of Triton X-100 after UV photodegradation. Environ Sci Pollut Res. 2021;28(9):11224–11232. doi: 10.1007/s11356-020-11362-2 [DOI] [PubMed] [Google Scholar]
- 67.Europe Union - Comission Regulation. COMMISSION REGULATION (EU) 2020/2160 of 18 December 2020 amending annex XIV to Regulation (EU) No 1907/2006 of the European Parliament and of the Council as regards the substance group 4-(1,1,3,3-Tetramethylbutyl)phenol, ethoxylated (covering well-define. Off J Eur Union. 2020;2:38–40. [Google Scholar]
- 68.Desai A, Vafaee T, Rooney P, et al. In vitro biomechanical and hydrodynamic characterisation of decellularised human pulmonary and aortic roots. J Mech Behav Biomed Mater. 2018;79(September 2017):53–63. doi: 10.1016/j.jmbbm.2017.09.019 [DOI] [PubMed] [Google Scholar]
- 69.Desai A, Ingham E, Berry HE, Fisher J, Jennings LM. The effect of decellularisation on the real time mechanical fatigue of porcine aortic heart valve roots. PLoS One. 2022;17(4 April):1–20. doi: 10.1371/journal.pone.0265763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Luo J, Korossis SA, Wilshaw S-P, Jennings LM, Fisher J, Ingham E. Development and characterization of acellular porcine pulmonary valve scaffolds for tissue engineering. Tissue Eng Part A. 2014;20(21–22):2963–2974. doi: 10.1089/ten.tea.2013.0573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Peró M, Casani L, Castells-Sala C, et al. Rabbit as an animal model for the study of biological grafts in pelvic floor dysfunctions. Sci Rep. 2021;11(1):1–9. doi: 10.1038/s41598-021-89698-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Castells-Sala C, Pérez ML, López-Chicón P, et al. Development of a full-thickness acellular dermal graft from human skin: case report of first patient rotator cuff patch augmentation repair. Transpl Immunol. 2023;78:101825. doi: 10.1016/j.trim.2023.101825 [DOI] [PubMed] [Google Scholar]
- 73.Prat-Vidal C, Rodríguez-Gómez L, Aylagas M, et al.. First-in-human PeriCord cardiac bioimplant: scalability and GMP manufacturing of an allogeneic engineered tissue graft. EBioMedicine. 2020;54. doi: 10.1016/j.ebiom.2020.102729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nieto-Nicolau N, López-Chicón P, Fariñas O, et al. Effective decellularization of human nerve matrix for regenerative medicine with a novel protocol. Cell Tissue Res. 2021;384(1):167–177. doi: 10.1007/s00441-020-03317-3 [DOI] [PubMed] [Google Scholar]