Abstract
Aims: To evaluate the usefulness of molecular markers in predicting histopathological and clinical response to preoperative high dose chemotherapy (HDCT) and survival of patients with advanced gastric cancer.
Methods: In a phase II trial, 25 patients with metastatic gastric cancer received preoperative tandem HDCT consisting of etoposide, cisplatin, and mitomycin, followed by autologous bone marrow transplantation to achieve surgical resectability. Samples before and after treatment, from normal and tumour tissue, were characterised histopathologically, and both p53 and BAX expression was analysed by immunohistochemistry. Pretreatment formalin fixed, paraffin wax embedded samples from normal and tumour tissue were microdissected, and the extracted DNA was preamplified using improved primer extension preamplification polymerase chain reaction. Detection of microsatellite instability (MSI) or loss of heterozygosity (LOH) was performed using markers for p53, BAX, BAT25, BAT26, D2S123, D17S250, and APC. Exons 5–9 of the p53 gene were sequenced directly on ABI 373.
Results: Four parameters were significantly associated with response to chemotherapy and prolonged overall survival: positive p53 immunostaining, positive p53 mutation status before chemotherapy, strong histological regression induced by preoperative HDCT, and surgical treatment. Patients’s sex or age, tumour location or stage, lymph node status, Lauren classification, MSI, or LOH did not influence duration of survival significantly in this high risk population.
Conclusion: Positive p53 immunostaining and p53 mutation status in pretreatment tumour biopsies might be useful molecular predictors of response and prognosis in patients with advanced gastric cancer treated by preoperative HDCT.
Keywords: gastric cancer, preoperative high dose chemotherapy, molecular parameters, histological regression, p53
Despite its decreasing prevalence, gastric carcinoma is still one of the major causes of cancer death worldwide.1 Because most tumours are diagnosed late in locally advanced stages, the overall median survival is only 16 months.2 Surgical treatment results in poor survival rates in advanced stages. Several studies have shown that preoperative chemotherapy can achieve a response in 50–60% of patients, and allows radical surgery in 40–50% of cases with previously unresectable tumours.3–8 To increase response rates and survival time, the benefit of high dose chemotherapy (HDCT) protocols with autologous bone marrow transplantation (ABMT) were evaluated for several cancer entities. HDCT is based on the hypothesis that increased dosage will overcome drug resistance, eradicate metastatic disease, and increase cure rates. Because of the notable toxicity of this treatment and the limited survival time of patients with advanced gastric cancer, it would be useful to be able to select those patients whose tumours will be sensitive to chemotherapy.9–11 Therefore, predictors for response to HDCT and survival would be clinically useful.
“Several studies have shown that preoperative chemotherapy can achieve a response in 50–60% of patients, and allows radical surgery in 40–50% of cases with previously unresectable tumours”
Clinical or histopathological parameters may be suitable. In addition, the characterisation of molecular alterations in tumour tissue may be useful to help predict response to chemotherapy.
The tumour suppressor protein p53 is a potent activator of apoptosis, and p53 dependent apoptosis modulates the cytotoxic effects of common antitumour agents such as 5-fluorouracil, doxorubicin, and cisplatin.12 p53 independent13 regulation of cisplatin induced tumour cell death includes expression of proteins of the Bcl-2 family,14 or activation of stress kinase cascades15,16 at the level of Bcl-2. Many apoptotic pathways converge, and the ratio of Bcl-2 to BAX protein might be the final determinant of whether a cell enters the execution phase.17 BAX is a dominant inhibitor of Bcl-2.18
It is well established that mismatch repair proteins are involved in triggering an apoptotic response after cisplatin damage.19 The mismatch repair protein complex can recognise and bind to 1,2–GpG adducts.20–22 Cells defective for mismatch repair become more resistant to G2 arrest and apoptosis provoked by cisplatin.23–26
The aim of our study was to assess the clinical, histopathological, and molecular parameters of patients with advanced gastric cancer treated by HDCT/ABMT, and their association with response to treatment and survival.
MATERIAL AND METHODS
Patients
Between October 1996 and December 2000, 25 patients with locally advanced gastric carcinoma were treated with a preoperative HDCT protocol followed by ABMT in a phase II study. All patients were deemed unresectable before the start of treatment, and tissue blocks from all patients were available for study. There were 11 women and 14 men, with a mean age of 50 years (range, 35–65).
A modified EAP schedule was used for induction treatment (doxorubicin, 40 mg/m2; etoposide, 120 mg/m2; cisplatin, 40 mg/m2), followed by granulocyte colony stimulating factor (filgrastim, Neupogen™) started at day 4 at a dose of 10 μ/kg body weight administered subcutaneously. Autologous peripheral blood stem cells were collected by leukapheresis after leucocyte nadir (Cobe Spectra; Cobe Laboratories, Lakewood, Colorado, USA) on consecutive days. If patients had stable disease following induction treatment, the first cycle of HDCT (etoposide, 500 mg/m2; cisplatin, 50 mg/m2; mitomycin C, 10 mg/m2; and BCNU (nitroso urea), 300 mg/m2) was administered three weeks thereafter. Patients who achieved a reduction in tumour size of > 50% after the first cycle of HDCT qualified for an identical second cycle four weeks later.
All patients were clinically judged as cT3 or cT4 according to the TNM classification,27 and had systemic metastasis. None of the patients had received systemic chemotherapy for gastric cancer previously. Response to chemotherapy was assessed by endoscopy, endosonography, and computed tomography scan, as defined by the Eastern Cooperation Oncology Group.28
Resectability and clinical response were defined as present if patients achieved partial remission (> 50% size reduction of the target lesion).
Definitive clinical TNM staging yielded 19 patient with T4 tumours and six with T3 tumours. N1, N2, and N3 disease was found in nine, three, and 13 cases, respectively. No patient showed N0 disease.
Response to chemotherapy enabled the resection of the tumour in 16 patients. Local (R0) resection was possible in three patients with T3, and nine with T4. In three patients with T4 tumours, resection margins were infiltrated by the tumour (R1 resection), and one patient with T3 disease underwent palliative resection only. All nine non-responders underwent non-curative palliative procedures.
Survival time was defined as duration between the date of first chemotherapy and the date of death or last follow up (table 1 ▶). Median survival of the studied 25 patients was 13 months. Median survival for patients with response to treatment and subsequent gastrectomy was 16 months (range, three to 50) and 7.5 months (range, five to 11.5) for those without resection. Twelve patients from the first group survived more than 12 months, and four are still alive.
Table 1.
Clinical and histopathological data, results of immunohistochemistry, p53 sequencing, and microsatellite analyses in 25 advanced gastric carcinomas
| Clinical data | |||||||||||||||||||||||||
| Patient ID | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 |
| Sex | f | f | m | m | f | m | f | f | m | f | m | f | m | f | m | m | f | f | f | m | m | m | m | m | m |
| Age at diagnosis (years) | 57 | 49 | 62 | 46 | 61 | 42 | 51 | 46 | 32 | 45 | 55 | 47 | 48 | 49 | 44 | 43 | 44 | 33 | 54 | 50 | 40 | 56 | 60 | 34 | 36 |
| Overall survival (months) | 12.3 | 5.5 | 50.1 | 17.7 | 20.8 | 6.2 | 8.1 | 7.4 | 9.5 | 11.8 | 12.6 | 4.9 | 14.5 | 12.3 | 4.2 | 13.3 | 5 | 29.6 | 6.9 | 12.6 | 3 | 16.7 | 11.6 | 19.7 | 4.7 |
| Survival status | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 |
| Resection | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 |
| Histopathological data | |||||||||||||||||||||||||
| Localisation | co | an | co/an | ca | co | co | an | an | an | co | an | co/an | co | co/an | an | ca | an | an | co | an | co | an | co | co | ca |
| Lauren classification | d | d | d | i | i | d | d | i | d | d | d | d | i | i | d | i | d | d | d | d | d | i | d | d | d |
| Histological regression | |||||||||||||||||||||||||
| Regression score | 0 | 0 | 3 | 3 | 3 | 0 | 0 | 0 | 0 | 3 | 2 | 1a | 1b | 2 | 1a | 1b | 0 | 3 | 0 | 2 | 2 | 3 | 0 | 3 | 0 |
| Immunohistochemistry | |||||||||||||||||||||||||
| p53 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 |
| BAX | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Microsatellite analysis | |||||||||||||||||||||||||
| Mononcleotide markers | |||||||||||||||||||||||||
| BAT25 | |||||||||||||||||||||||||
| BAT26 | |||||||||||||||||||||||||
| Dinucleotide markers | |||||||||||||||||||||||||
| APC | LOH | LOH | LOH | LOH | LOH | LOH | LOH | LOH | LOH | ||||||||||||||||
| D17S250 | LOH | ||||||||||||||||||||||||
| D2S123 | MSI | MSI | MSI | MSI | |||||||||||||||||||||
| Pentanucleotide marker | |||||||||||||||||||||||||
| TP53.Alk | LOH | LOH | LOH | LOH | |||||||||||||||||||||
| Sequencing results | |||||||||||||||||||||||||
| p53 mutation | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| Exon | 5 | 8 | 5 | 8 | 6 | 5 | 6 | 5 | |||||||||||||||||
| Codon | 177 | 282 | 162 | 273 | 213 | 175 | 220 | 178 | |||||||||||||||||
| Wild-type sequence | CCC | CGG | CGC | CGT | CGA | CGC | TAT | GCC | |||||||||||||||||
| Nucleotide substitution | TCC | CTG | CCC | TGT | CCA | CAC | TGT | GAC | |||||||||||||||||
| Allelic status of p53 mutation | het | het | het | het | het | het | het | het |
Sex: f, female; m, male. Survival status: 0, censored; 1, death. Localisation: ca, cardia; co, corpus; an, antrum. Histopathological classification according to Lauren29: i, intestinal type; d, diffuse type. Regression score according to the Japanese Research Society for Gastric Cancer classification of gastric carcinoma30: 0, grade 0; 1a, grade 1a; 1b, grade 1b; 2, grade 2; 3, grade 3. p53 immunohistochemistry: 0, negative; 1, positive (nuclear expression in >10% of tumour cells). BAX immunohistochemistry: 0, negative; 1, positive (cytoplasmatic expression in >5% of tumour cells). Allelic status of p53 mutation: het, heterozygeous; hemi, hemizygeous.
Table 1 ▶ summarises the clinical and histopathological data of all the patients.
Histopathological evaluation
Pretreatment biopsies and post-treatment specimens (16 gastrectomy specimens and nine diagnostic biopsies from non-resected patients after last chemotherapy) were evaluated by two pathologists (FB and PR). All tissue was fixed in 4% buffered formalin and embedded in paraffin wax. Histological sections were stained with haematoxylin and eosin; tumours were graded according to the World Health Organisation system31 and histologically classified according to the Lauren classification (table 1 ▶).29
The gastrectomy specimens or post-treatment biopsies (five biopsies with at least 18 sections from different tumour areas from each non-resected patient) were evaluated for histological changes resulting from preoperative chemotherapy according to the Japanese Research Society for Gastric Cancer classification.30
Immunohistochemistry
Immunohistochemical studies for the expression of p53 and BAX were performed on pretreatment tissue samples using an avidin–biotin peroxidase method with diaminobenzidine as chromogen. After antigen retrieval (microwave treatment of formalin fixed, paraffin wax embedded, 5 μm thick tissue sections for 40 minutes at 240 W in citrate buffer, pH 6.0), immunohistochemistry was carried out in a NEXES immunostainer (Ventana Medical System, Tucson, Arizona, USA), according to the manufacturer’s instructions. For p53, a mouse monoclonal antibody (Bp53–12; Santa Cruz Biotechnology, Santa Cruz, California, USA) was used at a dilution of 1/1000 as the primary antibody and for BAX immunostaining, a rabbit polyclonal IgG primary antibody (clone P-19; rabbit polyclonal IgG; Santa Cruz Biotechnology) was used. A positive reaction for p53 protein was defined as nuclear staining in > 10% of the tumour cell nuclei. A tumour was regarded as expressing BAX when more than > 5% of tumour cells had cytoplasmic staining.
The staining results were interpreted by one pathologist (FB) who was unaware of the clinical outcome of the patients.
Microdissection
Genomic DNA was prepared from 5 μm thick paraffin wax embedded sections of pretreatment tumour tissue after dewaxing and microdissection. Pure tumour cell populations were obtained using either manual microdissection with a needle under an inverted microscope (×40) or laser microdissection (PALM™ Robot Microbeam; PALM, Wolfratshausen, Germany), as described previously.32
DNA isolation
For genetic analysis, paired pretreatment normal and tumour DNA samples were extracted using the QIAmp DNA mini kit (Qiagen, Hilden, Germany), according to the protocol provided by the manufacturer. The purified DNA template was amplified by whole genome amplification33 before further polymerase chain reaction (PCR) analyses.34
Microsatellite analysis
Microsatellite analysis was performed using the recommended reference panel for detection of MSI in colorectal cancer. This panel is composed of two mononucleotide repeats (BAT25 and BAT26) and three dinucleotide repeats (D5S346, D2S123, and D17S250).35 PCR amplification was performed as described previously.36 Subsequently, PCR products were analysed by 6.7% polyacrylamide/50% urea gel electrophoresis in a SequiGen sequencing gel chamber (BioRad, Hercules, California, USA), followed by silver nitrate staining.37
All gels were evaluated independently by two observers (WD and FB). A tumour was classified as microsatellite unstable (MSI-H) if two or more markers showed instability.
LOH analysis (p53 ALK, D2S123, D17S250, and APC) was performed as described previously.38 Informative cases were scored as allelic losses when the intensity of a signal for an allele from tumour DNA was decreased to 50%, relative to the matched allele from normal DNA. All cases of LOH and MSI were verified in an independent PCR reaction.
p53 mutation
Exons 5–9 of the p53 tumour suppressor gene were sequenced directly using single exon amplification using an ABI 373 sequencer, as described previously.34
Statistics
Survival curves were constructed using the Kaplan–Meier method and differences were assessed by the log rank test. The χ2 test or Fisher’s exact test (two sided) was performed to determine the correlation between molecular features, clinicopathological parameters, and the survival of patients. A p value of < 0.05 was considered to be significant. All statistical analyses were conducted using SPSS software (SPSS, Chicago, Illinois, USA).
RESULTS
Parameters associated with response to chemotherapy and survival were positive p53 immunohistochemistry (p = 0.0003), positive p53 mutation status (p = 0.044), moderate to severe histological regression after chemotherapy (p = 0.002), and surgical treatment (p = 0.0007) (table 2 ▶). No associations were seen between response or survival time and sex, age, tumour location and stage, lymph node status, or Lauren classification.
Table 2.
Clinicopathological characteristics and their association with overall survival in 25 patients with metastatic gastric cancer
| Overall survival | ||||||
| Variable | Coding plan | N | Events | Censored | Survival (months) | Log rank |
| Surgery | 0 No surgery | 9 | 8 | 1 | 7.5 | 0.0007 |
| 1 Surgery | 16 | 13 | 3 | 16.0 | ||
| Histological regression | 0 No regression | 14 | 13 | 1 | 8.2 | 0.0018 |
| 1 Regression | 11 | 8 | 3 | 18.8 | ||
| p53 mutation | 0 No p53 mutation | 17 | 15 | 2 | 10.2 | 0.0439 |
| 1 p53 mutation | 8 | 6 | 2 | 18.5 | ||
| p53 immunohistochemistry | 0 Negative | 11 | 10 | 1 | 7.2 | 0.0003 |
| 1 Positive | 14 | 11 | 3 | 17.3 | ||
Regression score according to the Japanese Research Society for Gastric Cancer classification of gastric carcinoma30: 0, grade 0; 1a, grade 1a; 1b, grade 1b; 2, grade 2; 3, grade 3. p53 immunohistochemistry: 0, negative; 1, positive (nuclear expression in >10% of tumour cells).
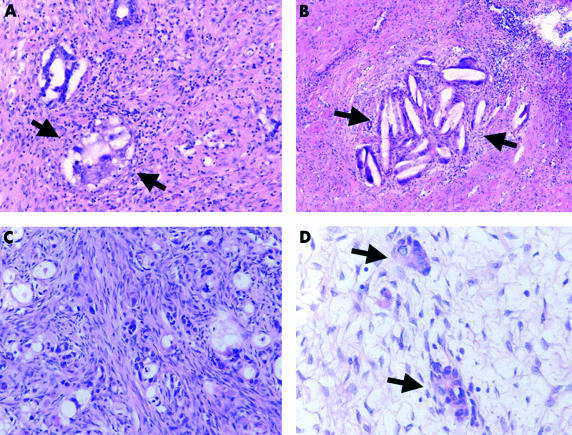
The histological effects of preoperative chemotherapy included degeneration of nuclei, coagulation necrosis of cancer cells, with or without formation of cholesterol crystals, appearance of giant cells with multiple nuclei, foamy degeneration of cancer cells, or creation of extracellular mucin pools without associated neoplastic cells (fig 1A–D ▶).
Figure 1.
Histological characteristics of tumour regression after chemotherapy. (A) foamy degeneration of cancer cells (arrows); original magnification, ×200; haematoxylin and eosin stained. (B) Coagulation necrosis of cancer cells with formation of cholesterol crystals (arrows); original magnification, ×100; haematoxylin and eosin stained. (C) Neoplastic cells with stromal fibrosis; original magnification, ×200; haematoxylin and eosin stained. (D) Residual neoplastic cells with stromal fibrosis (arrows); original magnification, ×400; haematoxylin and eosin stained.
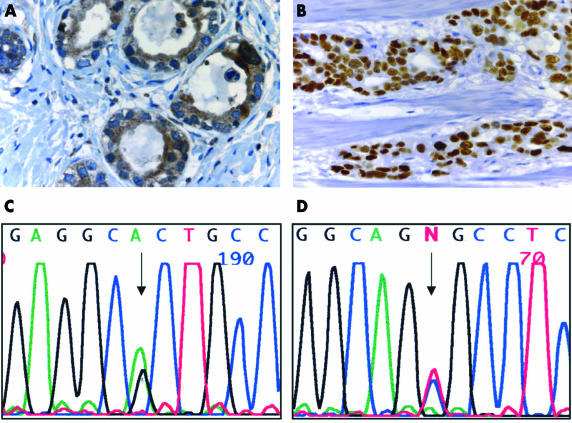
Histologically, response (grade 2 and 3) could be verified in 11 of the 25 tumours. There was no association with the Lauren classification or degree of differentiation. Nine of 12 responders revealed histological regression (p = 0.002). Despite our small sample size in this study, pretreatment p53 alterations were significantly associated with response to HDCT and longer survival. A high degree of p53 staining was detected in pretreatment biopsies in 14 of 25 patients (fig 2B ▶). There was a significant association between positive p53 immunostaining in pretreatment biopsies and a survival time longer than 12 months (p = 0.0003), pretreatment presence of p53 mutation (p = 0.0001), and histological regression (p = 0.002). p53 mutations were found in eight of the tumours (fig 2C,D ▶), and all of them were associated with p53 overexpression (p = 0.0001). In six of them, survival time was longer than 12 months (p = 0.044).
Figure 2.
Immunohistochemical and molecular characterisation of pretreatment biopsy specimens in patients with gastric cancer. (A) Strong cytoplasmic BAX expression in most of the cancer cells; original magnification, ×630. (B) Strong nuclear p53 expression in most of the cancer cells; original magnification, ×400. (C) p53 mutation in patient 13, exon 5, codon 175, CGC → CAC (5′ → 3′). (D) p53 mutation in patient 13, exon 5, codon 175, CGC → CAC (3′ → 5′).
Pretreatment positive BAX immunoreactivity was seen in 10 of the 25 patients (table 1 ▶; fig 2A ▶). There was no significant association with clinical and histological response to HDCT (p = 0.215).
Microsatellite analyses revealed low MSI (only one marker showing instability) in pretreatment biopsies in four of 25 tumours (table 1 ▶). None of the cases showed MSI-H or alterations at the mononucleotide markers BAT25 and BAT26.
LOH was detected most frequently at the APC locus (in nine of 25 patients), and the p53 locus (in four of 25 patient; table 1 ▶). Neither MSI nor LOH was associated with response to chemotherapy or survival.
DISCUSSION
Most patients with gastric cancer are unresectable at diagnosis, or will suffer a relapse after surgery, resulting in a five year overall survival of less than 20%.39–41 Preoperative chemotherapy could be an approach that might improve surgical resectability, which is one of the main prognostic factors in patients with gastric carcinoma.42 However, only 40–50% of patients benefit from this treatment modality, whereas approximately 30% of patients experience moderate to severe toxic side effects.6,8,43 Understanding the molecular genetic features that determine response or resistance to chemotherapy could permit the selection of the most suitable patients for preoperative treatment.
“Histological responders underwent surgical treatment in all cases and survived significantly longer than non-responders”
To test the hypothesis that dose escalation would provide a benefit with regard to resectability and overall survival, a phase II study was designed to determine the feasibility of etoposide, cisplatin, and mitomycin based HDCT followed by ABMT in a preoperative setting. In our study, we evaluate clinical, histological, and molecular markers and their associations with response to treatment and survival in this highly selected patient cohort.
Patients who underwent resection survived significantly longer than unresected patients. These data demonstrate that resectability after preoperative chemotherapy is prognostically beneficial for patients with advanced gastric cancer. However, the benefit of post-chemotherapeutic surgical treatment on the overall survival of the entire patient cohort could not be shown unequivocally, because only the patients with a clinical response underwent gastrectomy in our study.
Interestingly, the histological examination of gastrectomy specimens and biopsies obtained after chemotherapy could predict overall survival of the patients. Histological responders underwent surgical treatment in all cases and survived significantly longer than non-responders. This is in accordance with previous studies, which reported a trend towards decreased survival in patients with a poor histological response.44,45 However, it has to be considered that a detailed histopathological evaluation of histological regression of gastric cancer using several paraffin wax blocks of gastrectomy specimens is not entirely comparable to the findings investigating biopsies of patients with non-resectable cancer. To minimise the bias in this comparison, five biopsies with at least 18 sections for each biopsy were analysed.
The molecular markers analysed in this study included MSI, LOH at the p53 and APC loci, expression and mutation of the p53 tumour suppressor gene, and expression of the proapoptotic protein BAX.
Overexpression of p53 and the presence of p53 mutations in exons 5–9 in pretreatment biopsy specimens were significantly associated with increased overall survival. In addition, p53 protein expression and mutation status were the only clinical or molecular parameters associated with objective tumour regression and histological response.
Given the important role of wild-type p53 in apoptosis and the association of p53 mutations with poor survival in many tumour types,46 these results were surprising. In contrast to our results, p53 expression detected by immunohistochemistry in pretreatment endoscopic biopsies was associated with poor response to neoadjuvant cisplatin based chemotherapy in patients with gastric cancer in two studies.47,48 In a study by Kubicka et al,49 positive lymph nodes and p53 mutations were the only significant adverse prognostic markers for survival after curative resection for gastric cancer. However, there are also reports in which p53 overexpression and/or mutation is associated with better outcome after treatment of bladder cancer or glioblastoma.50,51
In our study, seven of the responding tumours, but only one of the non-responding tumours, showed mutations in exons 5–9 of the p53 gene. In addition, p53 LOH was found in the gastric cancers of eight of the responders and three of the non-responders. Two of the responders had both p53 mutation and p53 LOH. Fifty per cent of gastric cancers contain p53 mutations, and p53 LOH has been reported in 26–83% of cases.52 Sano et al found both LOH and p53 mutations in more than 60% of gastric tumours.53 In contrast, Kobayashi et al demonstrated cases of gastric cancer that displayed LOH and did not contain p53 mutations, and vice versa,54 which is similar to our findings. Ikeguchi et al described cisplatin induced apoptosis more frequently in gastric cancer cell lines with a wild-type p53 gene.55 In contrast, Grundei et al found that LOH at the p53 locus in advanced gastric cancer is associated with a good clinical response to cisplatin based neoadjuvant chemotherapy.56
A possible explanation is that p53 overexpression does not correlate with the inactivation status of the gene,57,58 or that we may have detected overexpression of the wild-type protein59 in a subset of patients. This could indicate an accelerated response to stress factors (such as DNA damage induced by cisplatin60), with subsequent effective tumour cell elimination by apoptosis. However, the association between p53 expression and mutational status on the one hand and between p53 mutations and prolonged overall survival on the other hand argues against this hypothesis.
p53 is known to be an important determinant of DNA damage induced apoptosis, and loss of p53 in tumours is associated with an unfavourable prognosis in many forms of cancer.61–63 Wild-type p53 is thought to render tumours more sensitive to treatment by the induction of apoptosis, and p53 inactivation may lead to resistance to treatment. However, because p53 is also responsible for prolonged cell cycle arrest after chemotherapy induced genetic damage, it is expected to facilitate DNA repair in the absence of an apoptotic response. Therefore, tumours that inactivate p53 during progression should be less capable of DNA repair and more sensitive to a DNA damage induced mitotic catastrophe. Specific mutant forms of p53 also confer a gain of function phenotype, manifested by augmented cell growth and tumorigenic potential. Blandino et al found that mutations at p53His175 and p53His179 provided substantial resistance to etoposide, whereas the protective effect of p53His273 and p53Trp248 was much milder.64 Mutant p53 can inhibit apoptosis after treatment of cancer cells with low drug concentrations (for example, cisplatin), but had no effect after the presence of high concentrations. These findings suggest a possible selective gain of function of mutant p53 that depends on the particular mutation and the identity and concentration of the chemotherapeutic drug. In summary, loss of p53, and possibly gain of function of the mutant p53 protein, might not lead to increased resistance of tumours to treatment, but could be a factor that contributes to sensitivity to chemotherapy.65
Microsatellite analyses revealed that none of the patients with advanced gastric cancers showed an MSI-H genotype. This is in agreement with the better prognosis of MSI positive gastric cancer,66 leading to an under-representation of MSI positive tumours in this highly selected patient cohort with advanced tumours.
“p53 protein expression and mutation status were the only clinical or molecular parameters associated with objective tumour regression and histological response”
Preoperative chemotherapy can increase the rate of curative resection in locally advanced gastric carcinoma. However, a significant response or survival advantage is only seen in less than half of patients treated.67 The only prognostic factors that are commonly accepted in gastric cancer are clinicopathological features, such as performance status, patient age, or macroscopic tumour type.68,69 The examination of molecular alterations on pretreatment biopsy samples may be of particular importance for identifying those patients who would be suitable for preoperative chemotherapy, in an attempt to achieve resectability, the main prognostic factor for survival in gastric cancer. Without appropriate predictive markers, patient selection is based on clinical staging parameters and results in the treatment of more than twice as many patients than will eventually benefit from such treatment.
Take home messages.
Positive p53 immunostaining, positive p53 mutation status before chemotherapy, strong histological regression induced by preoperative high dose chemotherapy (HDCT), and surgical treatment were significantly associated with response to chemotherapy and prolonged overall survival
Thus, positive p53 immunostaining and p53 mutation status in pretreatment tumour biopsies might be useful molecular predictors of response and prognosis in patients with advanced gastric cancer treated by preoperative HDCT
Clinical or biological markers that could predict reponse to such treatment would be extremely useful
Prospective follow up evaluation and investigation of additional molecular markers are warranted to define effective predictors for response to chemotherapy in patients with gastric cancer
The results of our study indicate that overexpression and mutation of p53 in pretreatment biopsy specimens could predict overall survival in patients with advanced gastric cancer receiving preoperative HDCT. The availability of clinical or biological markers to predict response to such treatment would be highly desirable. Prospective follow up evaluation and investigation of additional molecular markers are warranted to define effective predictors for response to chemotherapy in patients with gastric cancer.
Abbreviations
ABMT, autologous bone marrow transplantation
HDCT, high dose chemotherapy
LOH, loss of heterozygosity
MSI, microsatellite instability
MSI-H, microsatellite unstable
PCR, polymerase chain reaction
REFERENCES
- 1.Boring CC, Squires TS, Tong T, et al. Cancer statistics 1994. CA Cancer J Clin 1994;44:7–26. [DOI] [PubMed] [Google Scholar]
- 2.Siewert JR, Bottcher K, Stein HJ, et al. Relevant prognostic factors in gastric cancer: ten-year results of the German gastric cancer study. Ann Surg 1998;228:449–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilke H, Preusser P, Fink U, et al. New developments in the treatment of gastric carcinoma. Semin Oncol 1990;17(suppl 2):61–70. [PubMed] [Google Scholar]
- 4.Ajani JA, Roth JA, Ryan MB, et al. Intensive preoperative chemotherapy with colony-stimulating factor for resectable adenocarcinoma of the esophagus or gastroesophageal junction. J Clin Oncol 1993;11:22–8. [DOI] [PubMed] [Google Scholar]
- 5.Rougier Ph, Lasser Ph, Ducreux M, et al. Preoperative chemotherapy of locally advanced gastric cancer. Ann Oncol 1994;5(suppl 3):59–68. [DOI] [PubMed] [Google Scholar]
- 6.Kelsen D, Karpeh M, Schwartz G, et al. Neoadjuvant chemotherapy of high-risk gastric cancer: a phase II trial of preoperative FAMTX and postoperative intraperitoneal fluorouracil–cisplatin plus intravenous fluorouracil. J Clin Oncol 1996;14:1818–28. [DOI] [PubMed] [Google Scholar]
- 7.Melcher AA, Mort D, Maughan TS. Epirubicin, cisplatin and continuous infusion 5-fluorouracil (ECF) as neoadjuvant chemotherapy in gastroesophageal cancer. Br J Cancer 1996;74:1651–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelsen DP. Adjuvant and neoadjuvant therapy for gastric cancer. Semin Oncol 1996;23:379–89. [PubMed] [Google Scholar]
- 9.Tanigawa N, Morimoto H. Significance of surgical adjuvant chemotherapy for gastric cancer. J Surg Oncol 1991;46:203–7. [DOI] [PubMed] [Google Scholar]
- 10.Yamaue H, Tanimura H, Noguchi K, et al. Chemosensitivity testing of fresh human gastric cancer with highly purified tumor cells using the MTT assay. Br J Cancer 1992;66:794–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saikawa Y, Kubota T, Furukawa T, et al. Single-cell suspension assay with an MTT end point is useful for evaluating the optimal adjuvant chemotherapy for advanced gastric cancer. Jpn J Cancer Res 1994;85:762–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loewe SW, Ruley HE, Jacks T, et al. p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell 1993;74:957–67. [DOI] [PubMed] [Google Scholar]
- 13.Zamble DB, Jacks T, Lippard SJ. p53-dependent and -independent responses to cisplatin in mouse testicular teratocarcinoma cells. Proc Natl Acad Sci U S A 1998;95:6163–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strasser A, Harris AW, Jacks T, et al. DNA damage can induce apoptosis in proliferating lymphoid cells via p53-independent mechanisms inhibitable by Bcl-2. Cell 1994;79:329–39. [DOI] [PubMed] [Google Scholar]
- 15.Zanke BW, Boudreau K, Rubie E, et al. The stress-activated protein kinase pathway mediates cell death following injury induced by cis-platinum, UV irradiation or heat. Curr Biol 1996;6:606–13. [DOI] [PubMed] [Google Scholar]
- 16.Sanchez-Perez I, Murguia JR, Perona R. Cisplatin induces a persistent activation of JNK that is related to cell death. Oncogene 1998;16:533–40. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez VM, Fuertes MA, Alonso C, et al. Is cisplatin-induced cell death always produced by apoptosis? Mol Pharmacol 2001;59:657–63. [DOI] [PubMed] [Google Scholar]
- 18.Eliopoulos AG, Kerr DJ, Herod J, et al. The control of apoptosis and drug resistance in ovarian cancer: influence of p53 and Bcl-2. Oncogene 1995;11:1217–28. [PubMed] [Google Scholar]
- 19.Strathdee G, MacKean MJ, Illand M, et al. A role for methylation of the hMLH1 promoter in loss of hMLH1 expression and drug resistance in ovarian cancer. Oncogene 1999;18:2335–41. [DOI] [PubMed] [Google Scholar]
- 20.Mello JA, Acharya S, Fishel R, et al. The mismatch-repair protein hMSH2 binds selectively to DNA adducts of the anticancer drug cisplatin. Chem Biol 1996;3:579–89. [DOI] [PubMed] [Google Scholar]
- 21.Duckett DR, Drummond JT, Murchie AI, et al. Human MutS-α recognized damaged DNA base pairs containing O6-methylguanine, O4-methylthymine or the cisplatin-d(GpG) adduct. Proc Natl Acad Sci U S A 1996;93:6443–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamada M, O’Regan E, Brown R, et al. Selective recognition of a cisplatin–DNA adduct by human mismatch repair proteins. Nucleic Acids Res 1997;25:491–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drummond JT, Anthoney A, Brown R, et al. Cisplatin and adriamycin resistance are associated with MutLα and mismatch repair deficiency in an ovarian tumor cell line. J Biol Chem 1996;271:19645–8. [DOI] [PubMed] [Google Scholar]
- 24.Aebi S, Kurdi-Haidar B, Gordon R, et al. Loss of DNA mismatch repair in acquired resistance to cisplatin. Cancer Res 1996;56:3087–90. [PubMed] [Google Scholar]
- 25.Vaisman A, Varchenko M, Umar A, et al. The role of hMLH1, hMSH3 and hMSH6 defects in cisplatin and oxaliplatin resistance: correlation with replicative bypass of platinum–DNA adducts. Cancer Res 1998;58:3579–85. [PubMed] [Google Scholar]
- 26.Fink D, Nebel S, Norris PS, et al. Enrichment for DNA mismatch repair-deficient cells during treatment with cisplatin. Int J Cancer 1998;77:741–6. [DOI] [PubMed] [Google Scholar]
- 27.Hermanek P. Tumors of the gastrointestinal tract and the pancreas: histopathology, staging and prognosis. Anticancer Res 1999;19:2393–6. [PubMed] [Google Scholar]
- 28.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649–55. [PubMed] [Google Scholar]
- 29.Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. Acta Pathol Microbiol Scand 1965;64:31–49. [DOI] [PubMed] [Google Scholar]
- 30.Japanese Research Society for Gastric Cancer. Japanese classification of gastric carcinoma, 1st English ed. Tokyo: Kanehara, 1995.
- 31.Jass JR, Sobin LH, Watanabe H. The World Health Organization’s histologic classification of gastrointestinal tumors. A commentary on the second edition. Cancer 1990;15;66:2162–7. [DOI] [PubMed] [Google Scholar]
- 32.Wild P, Knuechel R, Dietmaier W, et al. Laser microdissection and microsatellite analyses of breast cancer reveal a high degree of tumor heterogeneity. Pathobiology 2000;68:180–90. [DOI] [PubMed] [Google Scholar]
- 33.Zhang L, Cui X, Schmitt K, et al. Whole genome amplification from a single cell: implications for genetic analysis. Proc Natl Acad Sci U S A 1992;89:5847–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dietmaier W, Hartmann A, Wallinger S, et al. Multiple mutation analyses in single tumor cells with improved whole genome amplification. Am J Pathol 1999;154:83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boland CR, Thibodeau SN, Hamilton SR, et al. A National Cancer Institute workshop on microsatellite instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res 1998;58:5248–57. [PubMed] [Google Scholar]
- 36.Hartmann A, Zanardo L, Bocker-Edmonston T, et al. Frequent microsatellite instability in sporadic tumors of the upper urinary tract. Cancer Res 2002;62:6796–802. [PubMed] [Google Scholar]
- 37.Schlegel J, Bocker T, Zirngibl H, et al. Detection of microsatellite instability in human colorectal carcinomas using a non-radioactive PCR-based screening technique. Virchows Arch 1995;426:223–7. [DOI] [PubMed] [Google Scholar]
- 38.Hartmann A, Rosner U, Schlake G, et al. Clonality and genetic divergence in multifocal low-grade superficial urothelial carcinomas as determined by chromosomes 9 and p53 deletion analysis. Lab Invest 2000;80:709–18. [DOI] [PubMed] [Google Scholar]
- 39.Ahlgren JD, Macdonald JS. Gastric cancer: epidemiology, pathology, detection, and staging. In: Macdonald JS, Hill MC, Roberts IM, eds.Gastrointestinal oncology. Philadelphia: JB Lippincott, 1992:151–8.
- 40.Boring CC, Squires TS, Tong T. Cancer statistics, 1993. C A Cancer J Clin 1993;43:7–26. [DOI] [PubMed] [Google Scholar]
- 41.Moriguchi S, Maehara Y, Korenaga D, et al. Prediction of survival time after curative surgery for advanced gastric cancer. Eur J Surg Oncol 1992;18:287–92. [PubMed] [Google Scholar]
- 42.Roder JD, Bottcher K, Siewert JR, et al. Prognostic factors in gastric carcinoma. Cancer 1993;72:2089–97. [DOI] [PubMed] [Google Scholar]
- 43.Rougier Ph, Lasser Ph, Ducreux M, et al. Preoperative chemotherapy of locally advanced gastric cancer. Ann Oncol 1994;5(suppl 3):59–68. [DOI] [PubMed] [Google Scholar]
- 44.Yonemura Y, Ooyama S, Matsumoto H, et al. Pancreaticoduodenectomy in combination with right hemicolectomy for surgical treatment of advanced gastric cancer located in the lower half of stomach. Int Surg 1991;76:226–9. [PubMed] [Google Scholar]
- 45.Yonemura Y, Katamyama K, Kamata T, et al. Surgical treatment of advanced gastric cancer with metastasis in para-aortic lymph node. Int Surg 1991;76:222–3. [PubMed] [Google Scholar]
- 46.Erenpreisa J, Cragg MS. Mitotic death: a mechanism of survival? A review. Cancer Cell Int 2002;1:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cascinu S, Graziano F, Del Ferro E, et al. Expression of p53 protein and resistance to preoperative chemotherapy in gastric cancer. Cancer 1998;83:1917–22. [PubMed] [Google Scholar]
- 48.Nakata B, Chung KH, Ogawa M, et al. p53 expression as a predictor of the response to chemotherapy in gastric cancer. Surg Today 1998;28:595–8. [DOI] [PubMed] [Google Scholar]
- 49.Kubicka S, Claas C, Staab S, et al. p53 mutation pattern and expression of c-erbB2 and c-met in gastric cancer: relation to histological subtypes, Helicobacter pylori infection, and prognosis. Dig Dis Sci 2002;47:114–21. [DOI] [PubMed] [Google Scholar]
- 50.Cote RJ, Esrig D, Groshen S, et al. p53 and treatment of bladder cancer. Nature 1997;358:123–5. [DOI] [PubMed] [Google Scholar]
- 51.Tada M, Matsumoto R, Iggo RD, et al. Selective sensitivity to radiation of cerebral glioblastomas harboring p53 mutations. Cancer Res 1998;58:1793–7. [PubMed] [Google Scholar]
- 52.Fenoglio-Preiser CM, Wang J, Stemmermann GN, et al. TP53 and gastric carcinoma: a review. Hum Mutat 2003;21:258–270. [DOI] [PubMed] [Google Scholar]
- 53.Sano T, Tsujino T, Yoshida K, et al. Frequent loss of heterozygosity on chromosomes 1q, 5q, and 17p in human gastric carcinomas. Cancer Res 1991;51:2926–31. [PubMed] [Google Scholar]
- 54.Kobayashi M, Kawashima A, Mai M, et al. Analysis of chromosome 17p13 (p53 locus) alterations in gastric carcinoma cells by dual color fluorescence in situ hybridization. Am J Pathol 1996;149:1575–84. [PMC free article] [PubMed] [Google Scholar]
- 55.Ikeguchi M, Tatebe S, Kaibara N, et al. Changes in levels of expression of p53 and the product of the bcl-2 in lines of gastric cancer cells during cisplatin-induced apoptosis. Eur Surg Res 1997;29:396–402. [DOI] [PubMed] [Google Scholar]
- 56.Grundei T, Vogelsang H, Ott K, et al. Loss of heterozygosity and microsatellite instability as predictive markers for neoadjuvant treatment in gastric carcinoma. Clin Cancer Res 2000;6:4782–8. [PubMed] [Google Scholar]
- 57.Hashimoto T, Tokuchi Y, Hayashi M, et al. P53 null mutations undetected by immunohistochemical staining predict a poor outcome with early-stage non-small cell lung carcinomas. Cancer Res 1999;59:5572–7. [PubMed] [Google Scholar]
- 58.Hartmann A, Blaszyk H, McGovern RM, et al. P53 mutations inside and outside of exons 5–8: the patterns differ in breast and other cancers. Oncogene 1995;16:681–8. [PubMed] [Google Scholar]
- 59.Hartwell LH, Kastan MB. Cell cycle control and cancer. Science 1994;266:1821–8. [DOI] [PubMed] [Google Scholar]
- 60.Pinto AL, Lippard SJ. Binding of the antitumor drug cis-diaminedichloroplatinum(II) (cisplatin) to DNA. Biochim Biophys Acta 1985;780:167–80. [DOI] [PubMed] [Google Scholar]
- 61.Cordon-Cardo C, Dalbagni G, Sarkis AS, et al. Genetic alterations associated with bladder cancer. Important Adv Oncol 1994;71–83. [PubMed]
- 62.Falette N, Paperin MP, Treilleux I, et al. Prognostic value of p53 gene mutations in a large series of node-negative breast cancer patients. Cancer Res 1998;58:1451–5. [PubMed] [Google Scholar]
- 63.Molina R, Segui MA, Climent MA, et al. p53 oncoprotein as a prognostic indicator in patients with breast cancer. Anticancer Res 1998;18:507–11. [PubMed] [Google Scholar]
- 64.Blandino G, Levine AJ, Oren M. Mutant p53 gain of function: differential effects of different p53 mutants on resistance of cultured cells to chemotherapy. Oncogene 1999;18:477–85. [DOI] [PubMed] [Google Scholar]
- 65.Gudkov AV, Komarova EA. The role of p53 in determining sensitivity to radiotherapy. Nat Rev Cancer 2003;3:117–29. [DOI] [PubMed] [Google Scholar]
- 66.Chiaravalli AM, Cornaggia M, Furlan D et al. The role of histological investigation in prognostic evaluation of advanced gastric cancer. Analysis of histological structure and molecular changes compared with invasive pattern and stage. Virchows Arch 2001;439:158–69. [DOI] [PubMed] [Google Scholar]
- 67.Ajani JA, Mansfield PF, Lynch PM, et al. Enhanced staging and all chemotherapy preoperatively in patients with potentially resectable gastric carcinoma. J Clin Oncol 1999;17:2403–11. [DOI] [PubMed] [Google Scholar]
- 68.Rougier P, Ducreux M, Mahjoubi M, et al. Efficacy of combined 5-fluorouracil and cisplatinum in advanced gastric carcinomas: a phase II trial with prognostic factor analysis. Eur J Cancer 1994;30:1263–9. [DOI] [PubMed] [Google Scholar]
- 69.Cascinu S, Labianca R, Alessandroni P, et al. Intensive weekly chemotherapy for advanced gastric cancer using fluorouracil, cisplatin, epi-doxorubicin, 6S-leucovorin, glutathione, and filgrastim: a report from the Italian Group for Digestive Tract Cancer. J Clin Oncol 1997;15:3313–19. [DOI] [PubMed] [Google Scholar]