Abstract
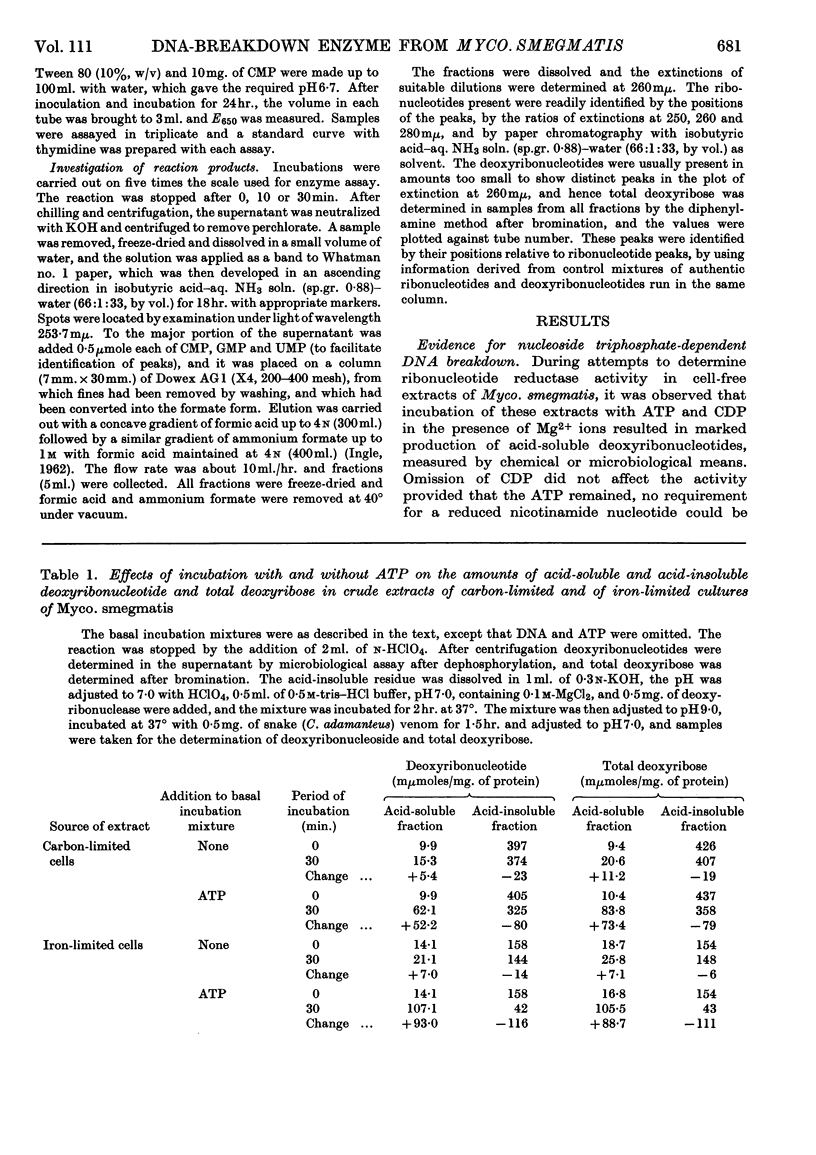
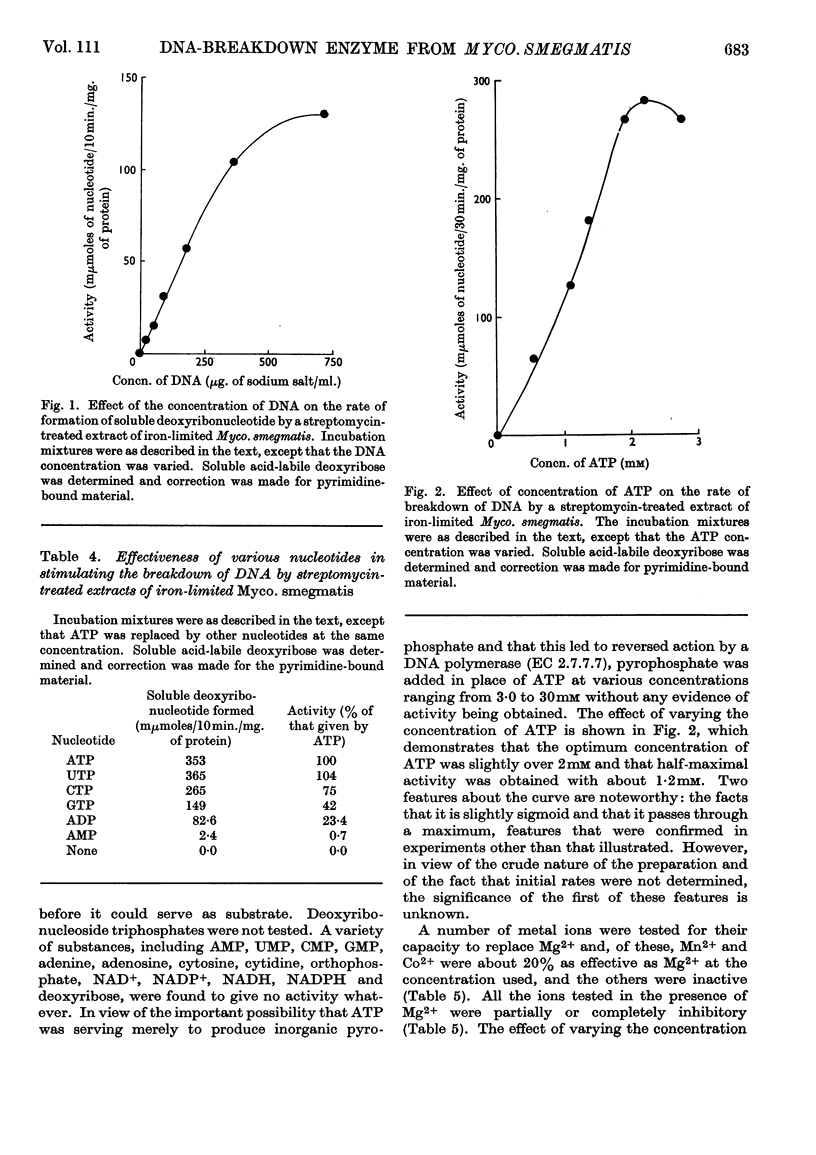
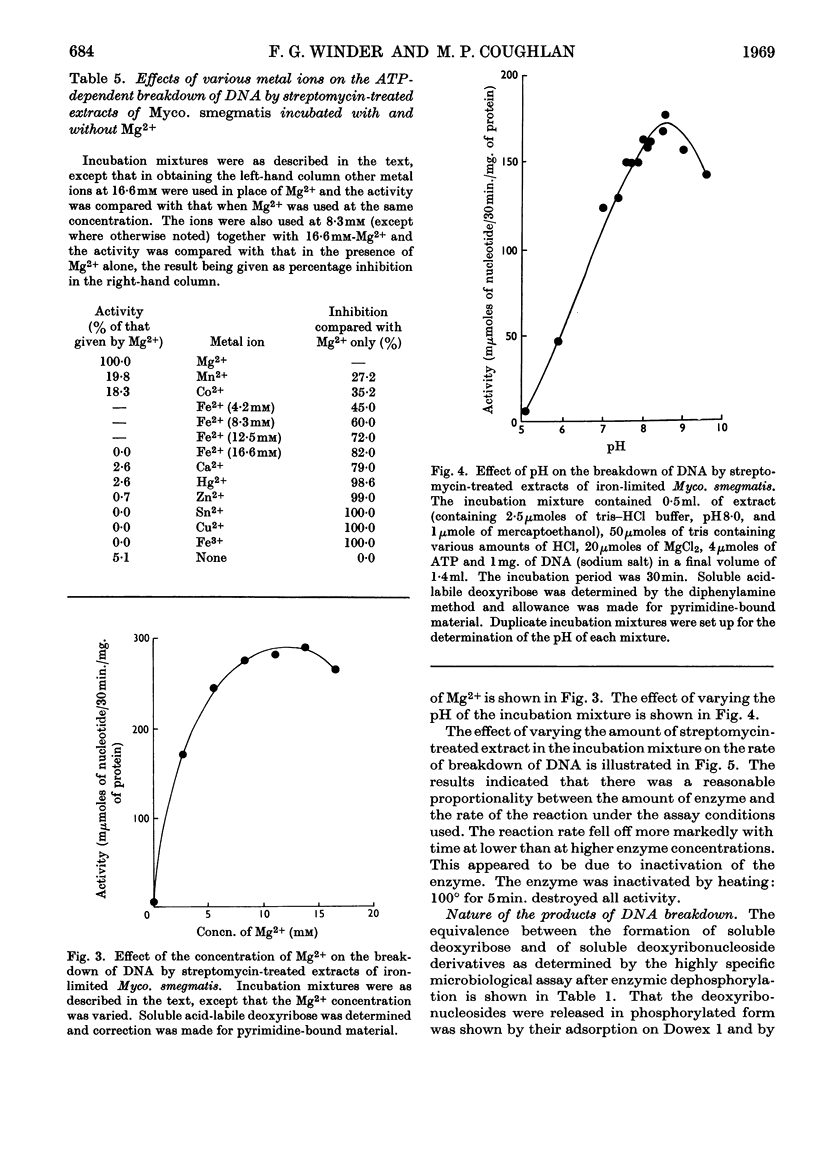
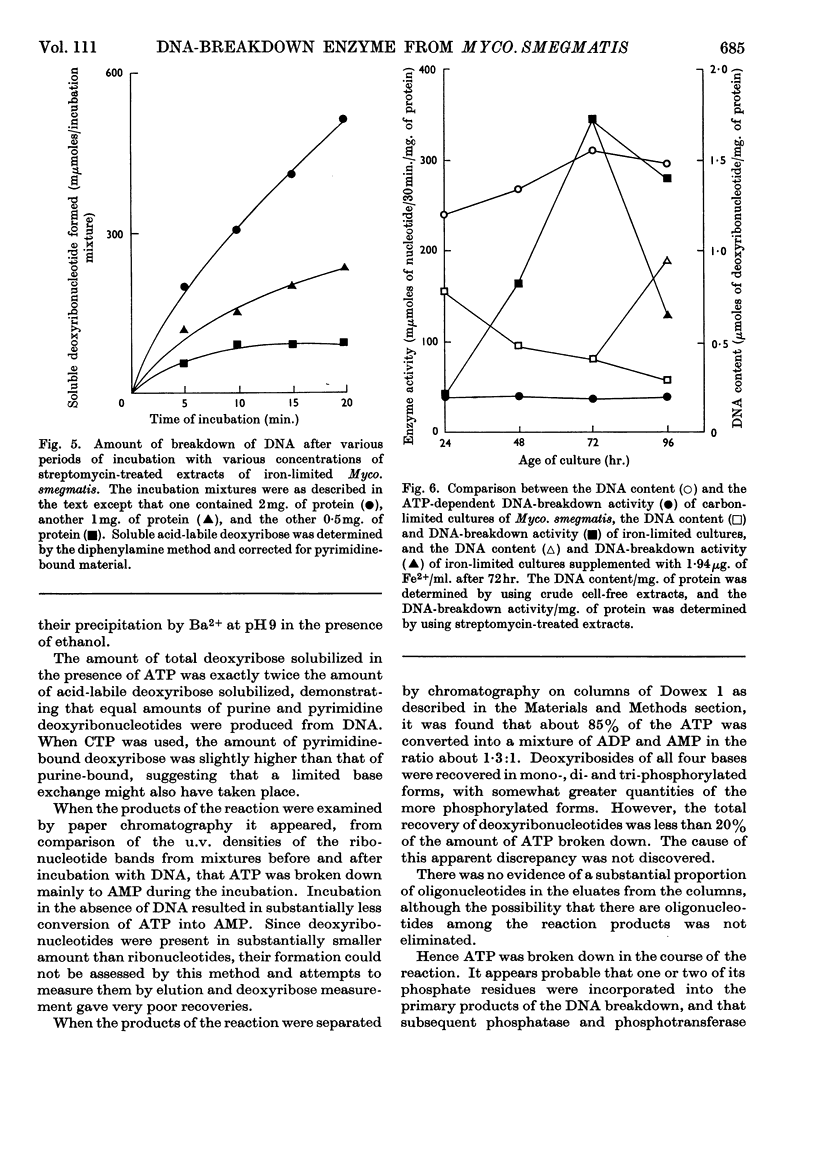
1. The presence of a nucleoside triphosphate-dependent DNA-breakdown system was demonstrated in extracts of Mycobacterium smegmatis. Its activity was increased substantially by iron limitation, apparently after the fall in DNA content that took place under these conditions. A maximal activity of about 0·2μmole of deoxyribonucleotide/30min./mg. of protein was found in crude extracts. 2. After slight purification by streptomycin treatment, the enzyme showed maximal activity with undenatured DNA (Km≃200μg./ml.), ATP (Km≃1·2mm) or UTP, CTP and GTP giving lower activity and pyrophosphate giving none, and Mg2+ ions (optimum concn. 12mm). The optimum pH was 8·5. 3. In the assay system there was proportionality between enzyme concentration and rate of reaction, but the rate fell off with time. 4. ATP was broken down in the reaction and monodeoxy-ribonucleotides were among the products, but the presence of some oligodeoxy-ribonucleotides was not excluded and the degree of phosphorylation of the primary products was uncertain.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anai M. [Deoxyribonuclease requiring nucleoside triphosphate]. Seikagaku. 1967 Mar;39(3):167–174. [PubMed] [Google Scholar]
- Blakley R. L. Cobamides and ribonucleotide reduction. II. Estimation of the enzymic formation of purine and pyrimidine deoxyribonucleotides by the use of the diphenylamine reagent. J Biol Chem. 1966 Jan 10;241(1):176–179. [PubMed] [Google Scholar]
- Brown N. C., Eliasson R., Reichard P., Thelander L. Nonheme iron as a cofactor in ribonucleotide reductase from E. coli. Biochem Biophys Res Commun. 1968 Mar 12;30(5):522–527. doi: 10.1016/0006-291x(68)90083-1. [DOI] [PubMed] [Google Scholar]
- Gottesman M. E., Canellakis E. S. The terminal nucleotidyltransferases of calf thymus nuclei. J Biol Chem. 1966 Oct 10;241(19):4339–4352. [PubMed] [Google Scholar]
- Kato K. I., Gonçalves J. M., Houts G. E., Bollum F. J. Deoxynucleotide-polymerizing enzymes of calf thymus gland. II. Properties of the terminal deoxynucleotidyltransferase. J Biol Chem. 1967 Jun 10;242(11):2780–2789. [PubMed] [Google Scholar]
- LARSSON A. ENZYMATIC SYNTHESIS OF DEOXYRIBONUCLEOTIDES. III. REDUCTION OF PURINE RIBONUCLEOTIDES WITH AN ENZYME SYSTEM FROM ESCHERICHIA COLI B. J Biol Chem. 1963 Oct;238:3414–3419. [PubMed] [Google Scholar]
- MASTER R. W. POSSIBLE SYNTHESIS OF POLYRIBONUCLEOTIDES OF KNOWN BASE-TRIPLET SEQUENCES. Nature. 1965 Apr 3;206:93–93. doi: 10.1038/206093b0. [DOI] [PubMed] [Google Scholar]
- MOORE E. C., HURLBERT R. B. Reduction of cytidine nucleotides to deoxycytidine nucleotides by mammalian enzymes. Biochim Biophys Acta. 1962 May 14;55:651–663. doi: 10.1016/0006-3002(62)90843-0. [DOI] [PubMed] [Google Scholar]
- MOORE E. C., REICHARD P. ENZYMATIC SYNTHESIS OF DEOXYRIBONUCLEOTIDES. VI. THE CYTIDINE DIPHOSPHATE REDUCTASE SYSTEM FROM NOVIKOFF HEPATOMA. J Biol Chem. 1964 Oct;239:3453–3456. [PubMed] [Google Scholar]
- Meselson M., Yuan R. DNA restriction enzyme from E. coli. Nature. 1968 Mar 23;217(5134):1110–1114. doi: 10.1038/2171110a0. [DOI] [PubMed] [Google Scholar]
- Novogrodsky A., Hurwitz J. The enzymatic phosphorylation of ribonucleic acid and deoxyribonucleic acid. I. Phosphorylation at 5'-hydroxyl termini. J Biol Chem. 1966 Jun 25;241(12):2923–2932. [PubMed] [Google Scholar]
- Olivera B. M., Lehman I. R. Linkage of polynucleotides through phosphodiester bonds by an enzyme from Escherichia coli. Proc Natl Acad Sci U S A. 1967 May;57(5):1426–1433. doi: 10.1073/pnas.57.5.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RATLEDGE C., WINDER F. G. The accumulation of salicylic acid by mycobacteria during growth on an iron-deficient medium. Biochem J. 1962 Sep;84:501–506. doi: 10.1042/bj0840501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSUDA Y., STRAUSS B. S. A DEOXYRIBONUCLEASE REACTION REQUIRING NUCLEOSIDE DI- OR TRIPHOSPHATES. Biochemistry. 1964 Nov;3:1678–1684. doi: 10.1021/bi00899a013. [DOI] [PubMed] [Google Scholar]
- WINDER F. G., O'HARA C. Effects of iron deficiency and of zinc deficiency on the composition of Mycobacterium smegmatis. Biochem J. 1962 Jan;82:98–108. doi: 10.1042/bj0820098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B., Richardson C. C. Enzymatic breakage and joining of deoxyribonucleic acid, I. Repair of single-strand breaks in DNA by an enzyme system from Escherichia coli infected with T4 bacteriophage. Proc Natl Acad Sci U S A. 1967 Apr;57(4):1021–1028. doi: 10.1073/pnas.57.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winder F. G., Coughlan M. P. The relationship between iron deficiency, deoxyribonucleic acid content and ribonucleoside triphosphate-dependent breakdown of deoxyribonucleic acid Mycobacterium smegmatis. Biochem J. 1967 Jun;103(3):67P–67P. [PMC free article] [PubMed] [Google Scholar]
- Zimmerman S. B., Little J. W., Oshinsky C. K., Gellert M. Enzymatic joining of DNA strands: a novel reaction of diphosphopyridine nucleotide. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1841–1848. doi: 10.1073/pnas.57.6.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]


