Abstract
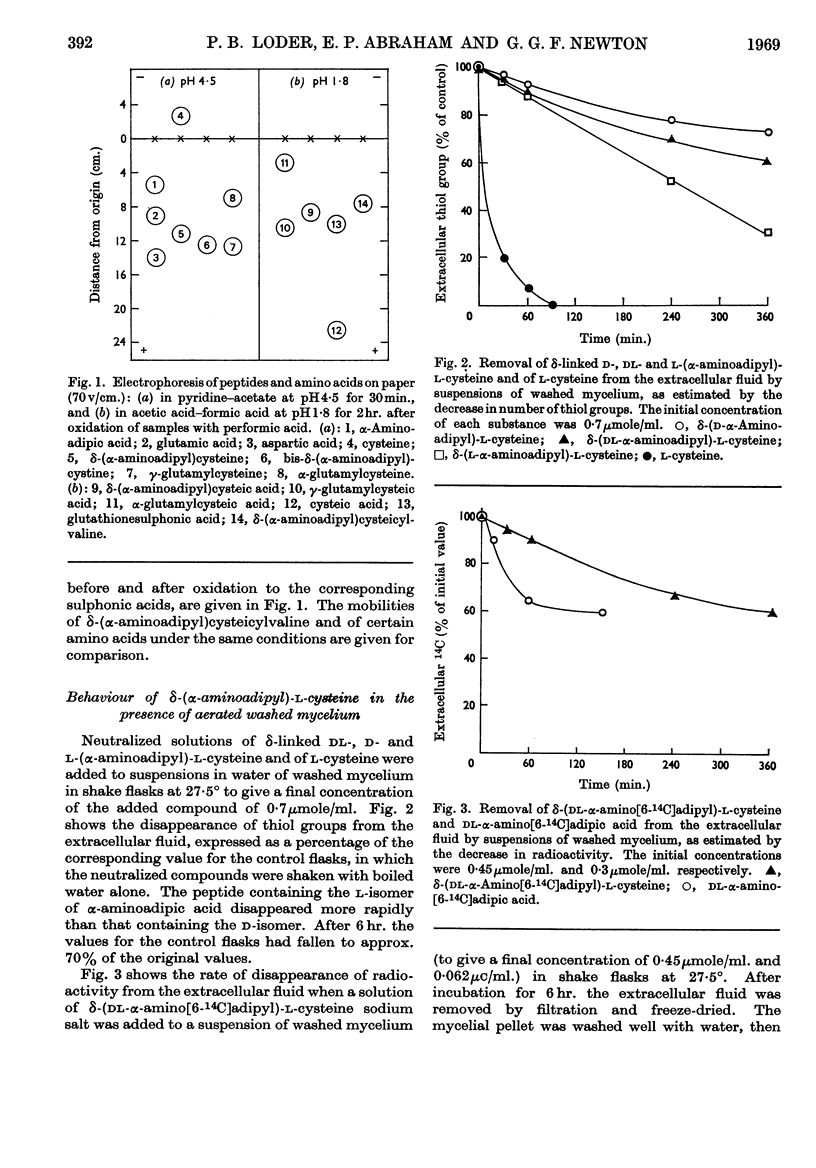
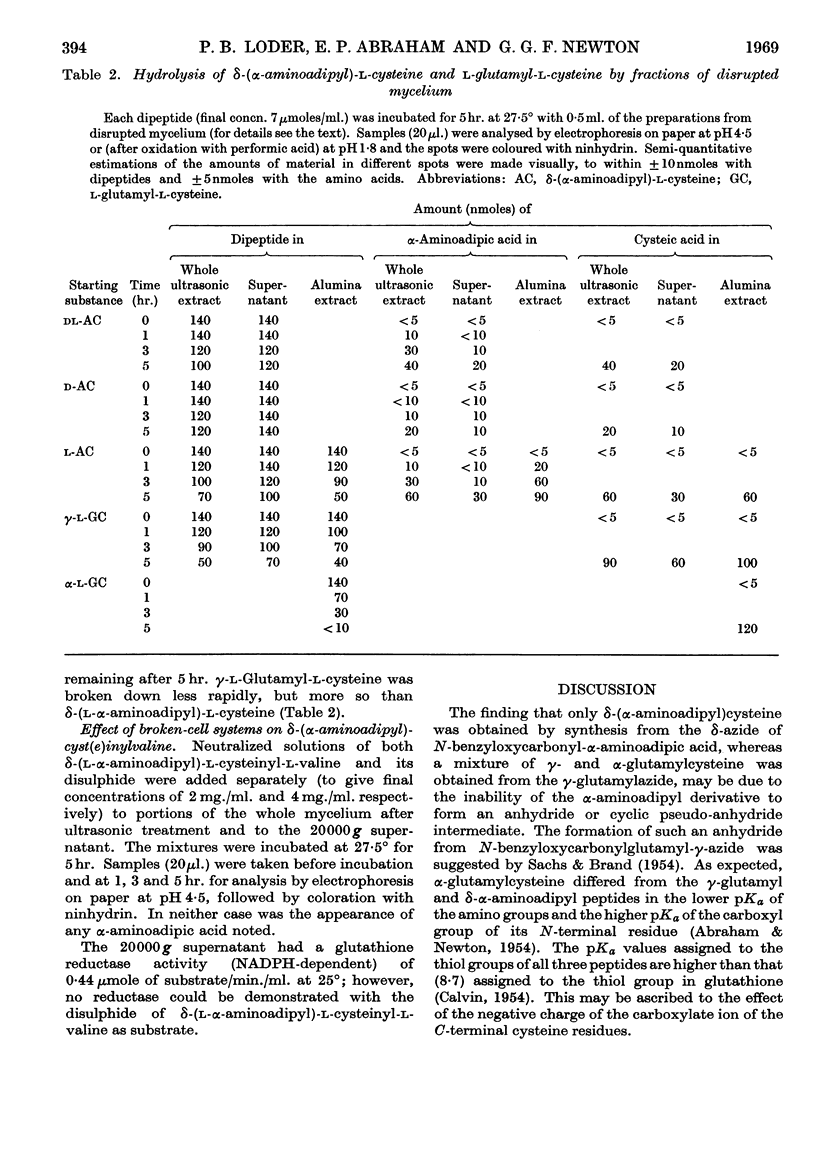
1. δ-(l-α-Aminoadipyl)-l-cysteine, the corresponding d- and dl-α-aminoadipyl isomers, δ-(dl-α-amino[6-14C]adipyl)-l-cysteine and γ- and α-l-glutamyl-l-cysteine were synthesized. 2. The behaviour of δ-(l-aminoadipyl)-l-cysteine and the corresponding d- and dl-α-aminoadipyl isomers was studied in the presence of suspensions of intact mycelium of a Cephalosporium sp., suspensions treated ultrasonically and extracts obtained by grinding with alumina. 3. With intact mycelium the l-α-aminoadipyl isomer was removed more rapidly from the extracellular fluid than the corresponding d-isomer. 4. Addition of δ-(dl-α-amino[6-14C]adipyl)-l-cysteine to suspensions of intact mycelium led to the labelling of extracellular and intracellular penicillin N and cephalosporin C, but also to extensive hydrolysis of the dipeptide. 5. Broken-cell systems hydrolysed δ-(l-α-aminoadipyl)-l-cysteine and the corresponding d-α-aminoadipyl isomer, but the former was hydrolysed more readily than the latter. 6. γ- and α-l-Glutamyl-l-cysteine were also hydrolysed but δ-(l-α-aminoadipyl)-l-cysteinyl-l-valine was not. 7. Only part of the enzyme activity in broken-cell systems responsible for the hydrolysis of δ-(α-aminoadipyl)-l-cysteine was present in the supernatant obtained on centrifugation at 20000g. 8. Possible implications of these findings are discussed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAHAM E. P., NEWTON G. G. Synthesis of D-delta-amino-delta-carboxyvalerylglycine (a degradation product of cephalosporin N) and of DL-delta-amino-delta-carboxyvaleramide. Biochem J. 1954 Oct;58(2):266–268. doi: 10.1042/bj0580266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEUTLER E., DURON O., KELLY B. M. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963 May;61:882–888. [PubMed] [Google Scholar]
- BREITENBACH J. W., DERKOSCH J., WESSELY F. Energetics of peptide formation. Nature. 1952 May 31;169(4309):922–922. doi: 10.1038/169922a0. [DOI] [PubMed] [Google Scholar]
- COLE M. PROPERTIES OF THE PENICILLIN DEACYLASE ENZYME OF ESCHERICHIA COLI. Nature. 1964 Aug 1;203:519–520. doi: 10.1038/203519a0. [DOI] [PubMed] [Google Scholar]
- DEMAIN A. L. SYNTHESIS OF CEPHALOSPORIN C BY RESTING CELLS OF CEPHALOSPORIUM SP. Clin Med (Northfield) 1963 Nov;70:2045–2051. [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Harington C. R., Rivers R. V. The synthesis of cysteine-(cystine-) tyrosine peptides and the action thereon of crystalline pepsin. Biochem J. 1944;38(5):417–423. doi: 10.1042/bj0380417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAUFMANN W., BAUER K. VARIETY OF SUBSTRATES FOR A BACTERIAL BENZYL PENICILLIN-SPLITTING ENZYME. Nature. 1964 Aug 1;203:520–520. doi: 10.1038/203520a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Smith B., Warren S. C., Newton G. G., Abraham E. P. Biosynthesis of penicillin N and cephalosporin C. Antibiotic production and other features of the metabolism of Cephalosporium sp. Biochem J. 1967 Jun;103(3):877–890. doi: 10.1042/bj1030877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneath P. H., Collins J. F. A method for the chromatographic detection of penicillins and related compounds and of penicillinase. Biochem J. 1961 Jun;79(3):512–514. doi: 10.1042/bj0790512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TROWN P. W., SMITH B., ABRAHAM E. P. Biosynthesis of cephalosporin C from amino acids. Biochem J. 1963 Feb;86:284–291. doi: 10.1042/bj0860284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren S. C., Newton G. G., Abraham E. P. Use of alpha-aminoadipic acid for the biosynthesis of penicillin N and cephalosporin C by a Cephalosporium sp. Biochem J. 1967 Jun;103(3):891–901. doi: 10.1042/bj1030891. [DOI] [PMC free article] [PubMed] [Google Scholar]


