Abstract
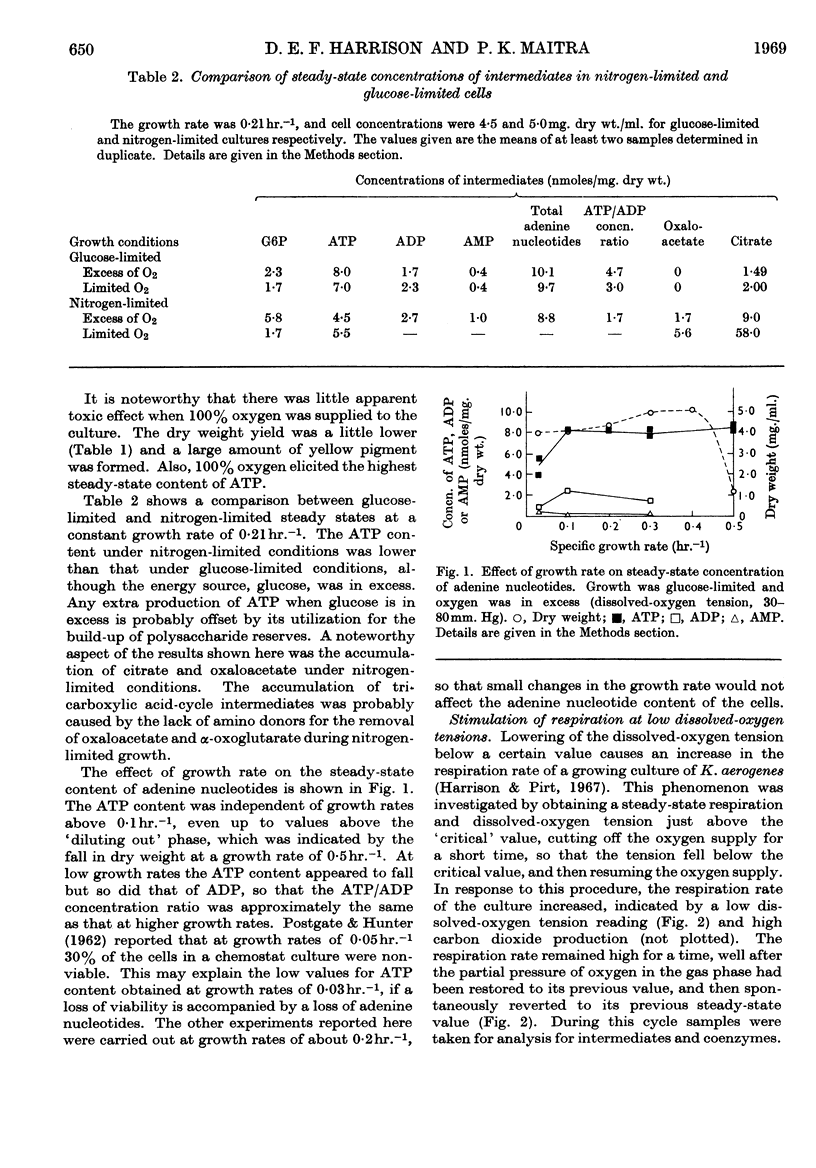
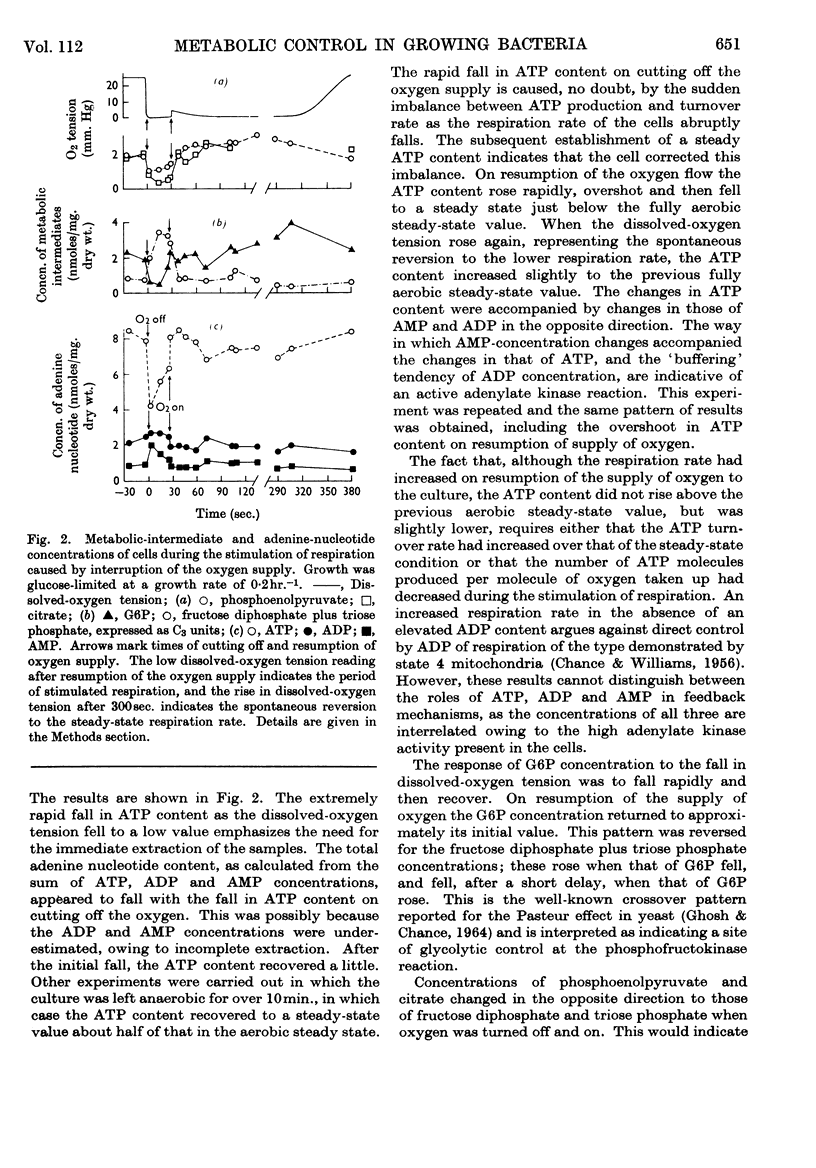
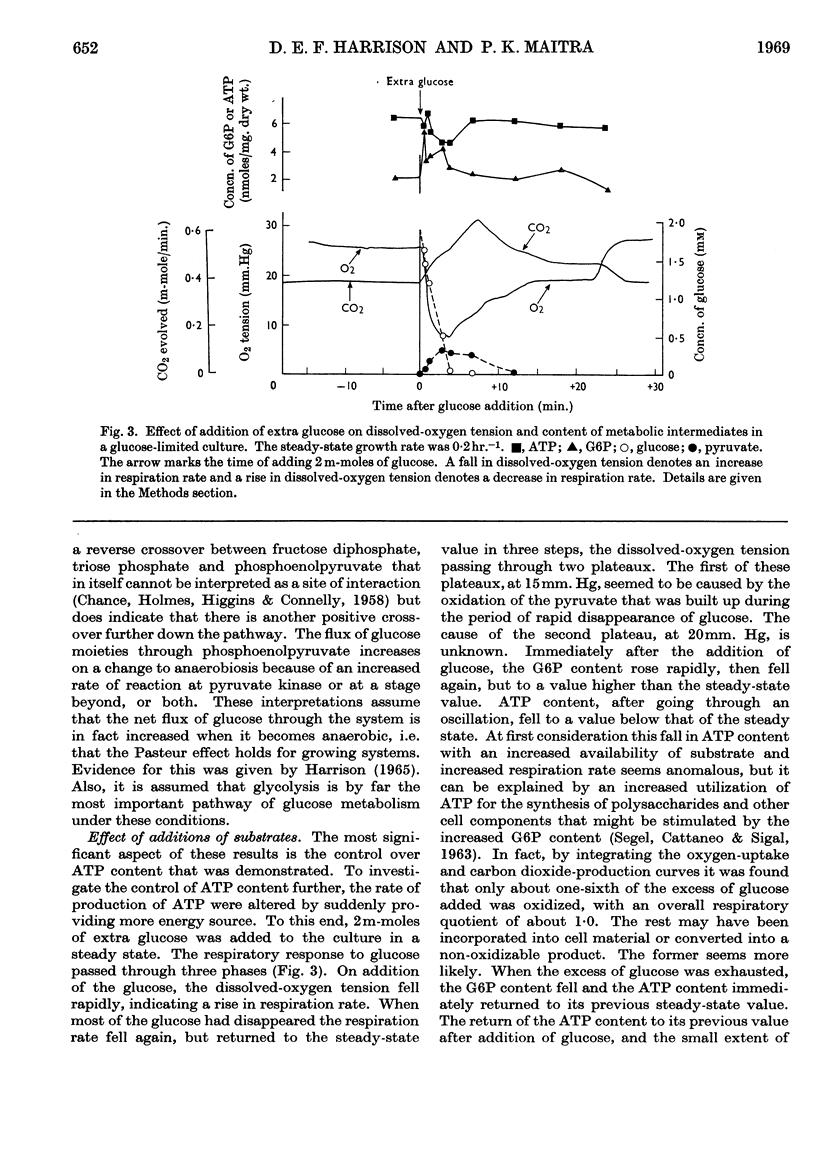
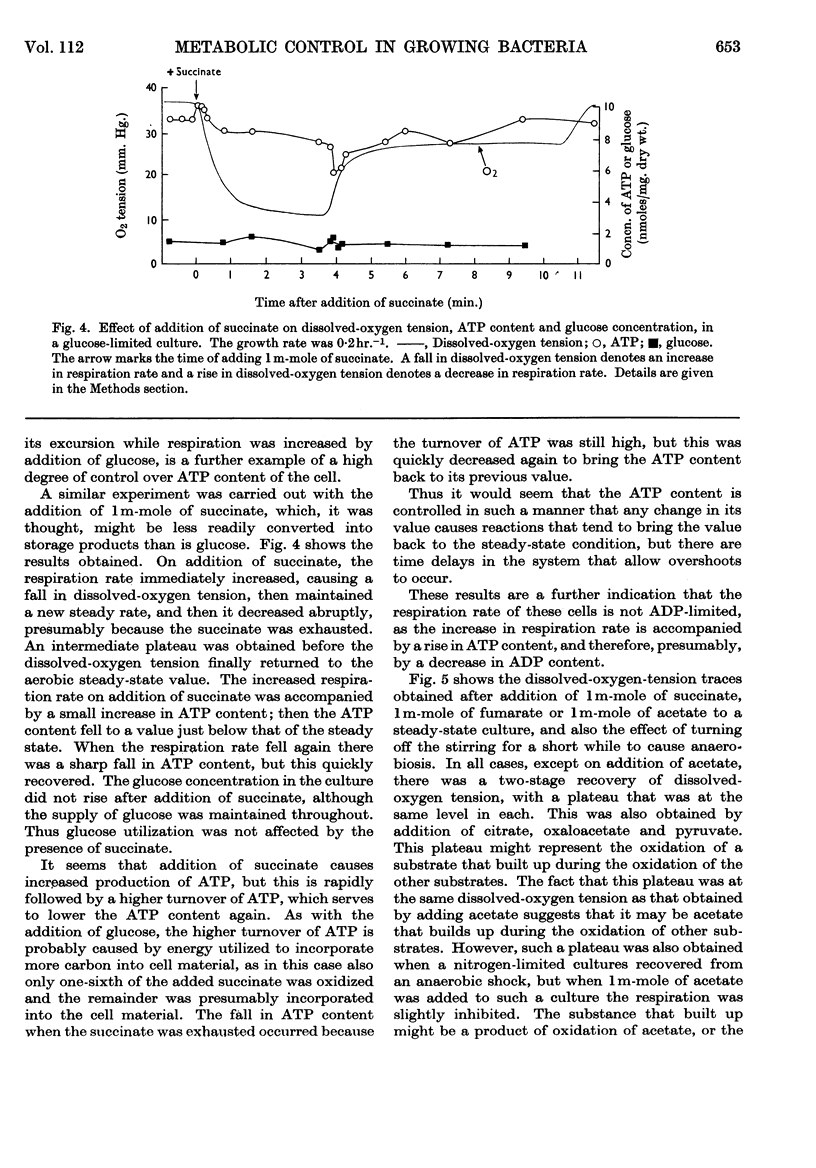
1. A rapid-sampling technique was used to obtain perchloric acid extracts of cells growing in a chemostat culture, so that meaningful values for ATP content could be obtained in spite of the fact that the turnover time for the total ATP content was about 1sec. 2. For steady-state growth, it was found that, in a glucose-limited chemostat culture, the ATP/ADP concentration ratio was approximately constant with changes in dissolved-oxygen tensions above the critical value, but fell when the culture was grown under oxygen-limited conditions and was at a minimum in anaerobically grown cultures. The steady-state ATP content was lower in cells growing under nitrogen-limited conditions with glucose in excess than in glucose-limited cells. The steady-state ATP content was independent of growth rate at growth rates over 0·1hr.−1. 3. When the respiration rate of the cells was stimulated by lowering the oxygen tension the ATP content did not increase, indicating either an increased turnover rate of ATP or a fall in the P/O ratio. The sudden addition of extra glucose or succinate to a glucose-limited culture increased the respiration rate of the cells, but the ATP content quickly returned to the steady-state value after initial perturbations. This control over ATP content is explained in terms of regulation by adenine nucleotides of the catabolism and anabolism of glucose. An exception to this control over ATP content was found when the respiration rate was stimulated by addition of an antifoam.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAUCHOP T., ELSDEN S. R. The growth of micro-organisms in relation to their energy supply. J Gen Microbiol. 1960 Dec;23:457–469. doi: 10.1099/00221287-23-3-457. [DOI] [PubMed] [Google Scholar]
- CHANCE B. FEEDBACK CONTROL OF METABOLISM IN ASCITES TUMOR CELLS. Acta Unio Int Contra Cancrum. 1964;20:1028–1032. [PubMed] [Google Scholar]
- CHANCE B., GHOSH A., HIGGINS J. J., MAITRA P. K. CYCLIC AND OSCILLATORY RESPONSES OF METABOLIC PATHWAYS INVOLVING CHEMICAL FEEDBACK AND THEIR COMPUTER REPRESENTATIONS. Ann N Y Acad Sci. 1964 Jul 31;115:1010–1024. [PubMed] [Google Scholar]
- CHANCE B., HOLMES W., HIGGINS J., CONNELLY C. M. Localization of interaction sites in multi-component transfer systems: theorems derived from analogues. Nature. 1958 Nov 1;182(4644):1190–1193. doi: 10.1038/1821190a0. [DOI] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- Cole H. A., Wimpenny J. W., Hughes D. E. The ATP pool in Escherichia coli. I. Measurement of the pool using modified luciferase assay. Biochim Biophys Acta. 1967;143(3):445–453. doi: 10.1016/0005-2728(67)90050-3. [DOI] [PubMed] [Google Scholar]
- ESTABROOK R. W., MAITRA P. K. A fluorimetric method for the quantitative microanalysis of adenine and pyridine nucleotides. Anal Biochem. 1962 May;3:369–382. doi: 10.1016/0003-2697(62)90065-9. [DOI] [PubMed] [Google Scholar]
- FRANZEN J. S., BINKLEY S. B. Comparison of the acid-soluble nucleotides in Escherichia coli at different growth rates. J Biol Chem. 1961 Feb;236:515–519. [PubMed] [Google Scholar]
- Forrest W. W. Adenosine triphosphate pool during the growth cycle in Streptococcus faecalis. J Bacteriol. 1965 Oct;90(4):1013–1018. doi: 10.1128/jb.90.4.1013-1018.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A., Chance B. Oscillations of glycolytic intermediates in yeast cells. Biochem Biophys Res Commun. 1964 Jun 1;16(2):174–181. doi: 10.1016/0006-291x(64)90357-2. [DOI] [PubMed] [Google Scholar]
- Harrison D. E., Pirt S. J. The influence of dissolved oxygen concentration on the respiration and glucose metabolism of Klebsiella aerogenes during growth. J Gen Microbiol. 1967 Feb;46(2):193–211. doi: 10.1099/00221287-46-2-193. [DOI] [PubMed] [Google Scholar]
- Hempfling W. P., Höfer M., Harris E. J., Pressman B. C. Correlation between changes in metabolite concentrations and rate of ion transport following glucose addition to Escherichia coli B. Biochim Biophys Acta. 1967 Jul 25;141(2):391–400. doi: 10.1016/0304-4165(67)90114-6. [DOI] [PubMed] [Google Scholar]
- MAITRA P. K., ESTABROOK R. W. A FLUOROMETRIC METHOD FOR THE ENZYMIC DETERMINATION OF GLYCOLYTIC INTERMEDIATES. Anal Biochem. 1964 Apr;7:472–484. doi: 10.1016/0003-2697(64)90156-3. [DOI] [PubMed] [Google Scholar]
- Maitra P. K., Estabrook R. W. Studies of baker's yeast metabolism. II. The role of adenine nucleotides and inorganic phosphate in the control of respiration during alcohol oxidation. Arch Biochem Biophys. 1967 Jul;121(1):129–139. doi: 10.1016/0003-9861(67)90017-3. [DOI] [PubMed] [Google Scholar]
- POSTGATE J. R., HUNTER J. R. The survival of starved bacteria. J Gen Microbiol. 1962 Oct;29:233–263. doi: 10.1099/00221287-29-2-233. [DOI] [PubMed] [Google Scholar]
- Polakis E. S., Bartley W. Changes in the intracellular concentrations of adenosine phosphates and nicotinamide nucleotides during the aerobic growth cycle of yeast on different carbon sources. Biochem J. 1966 Jun;99(3):521–533. doi: 10.1042/bj0990521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH R. C., MAALOE O. EFFECT OF GROWTH RATE ON THE ACID-SOLUBLE NUCLEOTIDE COMPOSITION OF SALMONELLA TYPHIMURIUM. Biochim Biophys Acta. 1964 May 11;86:229–234. doi: 10.1016/0304-4165(64)90047-9. [DOI] [PubMed] [Google Scholar]


