Abstract
Infertility is a primary health issue affecting about 15% of couples of reproductive ages worldwide, leading to physical, mental, and social challenges. Advances in nanobiotechnology and regenerative medicine are opening new therapeutic horizons for infertility by developing polysaccharide-based nanostructured biomaterials. This review explores the role of tissue engineering and regenerative medicine in infertility treatment, explicitly focusing on the promising potential of polysaccharide-based hydrogels. In this context, using these biomaterials offers unique advantages, including biodegradability, biocompatibility, and the ability to mimic the natural endometrial microenvironment, making them highly effective for applications in endometrial regeneration, ovarian tissue engineering, spermatogenesis support, and controlled drug delivery. This review discusses the various properties and uses of polysaccharide-based hydrogels, like alginate, hyaluronic acid, and chitosan, in helping to restore reproductive function. While these materials hold great promise, some notable challenges to their clinical use include issues like rapid degradation, mechanical instability, and potential immune reactions. Future research should focus on developing hybrid hydrogels, investigating advanced fabrication techniques, and testing these materials in clinical settings. By combining findings from recent studies, this review aims to provide a solid foundation for researchers and clinicians looking to discover new and effective strategies for treating infertility, ultimately connecting research efforts with practical applications in healthcare.
Graphical Abstract
Keywords: Infertility, Regenerative medicine, Tissue engineering, Polysaccharide, Hydrogel
Introduction
Infertility is one of the worldwide health issues, impacting around 15% of couples of reproductive ages. It can be a severe problem affecting a person’s physical and mental well-being and their social life, mainly for younger couples [1, 2]. While infertility does not threaten individual lives, its impact can be felt in all areas of personal and social life. Infertility can lead to psychological challenges such as depression and anxiety and negatively impact relationships and social life. Health issues in the male and female reproductive systems, which can arise from illnesses, injuries, or treatments like those for cancer, may result in infertility.
Women’s health issues, especially reproductive health, receive less attention in scientific research. One of the reasons for this problem is the insufficient understanding of the essential function of the uterus, which limits the development of effective treatments for female infertility [3]. The inner lining of the female’s uterus, known as the endometrium, is a tissue that undergoes dynamic regeneration [4, 5]. In a regular menstrual cycle, where pregnancy does not occur, the part of the shed endometrium is repaired without scarring [5]. On the opposite side, intrauterine surgery can damage the uterine endometrium, potentially leading to infertility. However, conventional medical treatments are usually unsuccessful in achieving the desired results and pregnancy [5]. Also, in intrauterine adhesions (IUA), which is the most common type of endometrial damage, fibrotic tissue replaces the endometrium, and this condition is known as “Asherman’s syndrome” (AS) [5]. In this case, the endometrium becomes thin and no longer reacts to estrogen and progesterone [6]. Currently, uterine adhesions are commonly treated with traditional methods, such as hormone therapy. However, the results of these treatments are poor in severe cases [7, 8].
On the other hand, various factors can contribute to infertility, but studies show that more than half of infertility cases (about 70%) are due to factors related to men [2]. Men can experience infertility due to various factors, including ejaculatory disorders, chromosomal disorders, and varicoceles. Moreover, certain illnesses can result in the loss of germ cells and the deletion of essential genes on the Y chromosome. Surgical and hormonal treatments are often used to address male infertility, but they don’t always succeed for everyone. In such cases, assisted reproductive techniques (ART) can be a helpful alternative for treating infertility [9]. Currently, regenerative medicine and the use of scaffolds have been offered as promising approaches to treating these abnormalities. These methods are considered innovative and effective solutions, especially for young couples facing infertility [1].
In this context, with the growth and development of science, the use of biomaterials and nano-bio scaffolds for drug delivery to repair endometrial injuries and infertility-related abnormalities has increased [6, 10]. Biomaterials serve as effective carriers by mimicking the structure of natural endometrial tissue and releasing growth factors and drugs in a controlled manner [5]. One of these water-soluble, non-toxic, and bioactive biological materials is polysaccharides, which are composed of monosaccharide units and are used to prepare hydrogels due to their biodegradability, high biocompatibility, and low cost [11–13]. Moreover, polysaccharide-based hydrogels have a sponge-like structure that enables the trapping of enzymes, aptamers, antibodies, and various other molecules inside their framework and on their surface. These hydrogels can simulate the characteristics of different tissues of the body. For this reason, researchers have studied them as nanostructure scaffolds and biological inks to engineer tissues and produce various types of organs [12, 14, 15]. In the field of infertility treatment, polysaccharide-based hydrogels have unique advantages. Their ability to mimic the extracellular matrix (ECM) and provide a mimetic environment supports cell attachment, proliferation, and differentiation—key factors for successful tissue engineering [16]. For example, these hydrogels have been used as scaffolds to regenerate damaged endometrial tissue, promote uterine healing, and facilitate embryo implantation [17–19]. Furthermore, their ability to provide a controlled release of bioactive agents, such as growth factors and reproductive hormones, increases their potential to create a supportive microenvironment for fertility restoration [20–22].
So, polysaccharide-based hydrogels improve cellular interactions and enable controlled drug delivery, making them ideal for tissue engineering applications. Additionally, recent advances in 3D bio-printing further enhance their potential, allowing the creation of complex and patient-specific constructs for reproductive health applications. These innovations represent a significant shift in infertility treatment, moving towards personalized and less invasive solutions.
Researchers have explored polysaccharide-based hydrogels in various biomedical fields, but they have not widely considered their application in infertility treatment. This article reviews selected studies to highlight the potential of these hydrogels in addressing infertility-related conditions. It illuminates their promise as innovative materials for infertility treatment by examining their role in mimicking natural reproductive tissues, enabling controlled drug delivery, and supporting regenerative programs. This article provides a comprehensive analysis of polysaccharide-based hydrogels in treating infertility, highlighting their properties, applications, and limitations. This focus addresses a topic where extensive reviews are often lacking.
This review focuses on a crucial question: Can polysaccharide-based hydrogels be effective in treating infertility? It investigates whether polysaccharide-based hydrogels can serve as effective alternatives to current treatments and identifies which types are more ideal for specific conditions. The objectives are to summarize current findings, assess the potential and limitations of different hydrogels, and suggest future research paths to enhance their clinical applications. By examining their applications in tissue engineering, drug delivery, and regenerative medicine, this review demonstrates how they can create bioactive environments for tissue repair and regeneration, ultimately advancing infertility treatments and improving reproductive health care.
Cause and impact of infertility in men and women
Infertility is a critical concern in gynecology, indicated by the inability to conceive following one year of engaging in unprotected sexual relations [23]. The World Health Organization (WHO) recognizes infertility as a serious public health issue that affects people worldwide [24]. It categorizes infertility into primary and secondary types based on whether a person has been pregnant before [25]. Both female and male factors are essential in understanding the causes of infertility. Prevalent etiologies of female infertility include disorders related to menstruation and ovulation, as well as issues with the uterus [25]. In males, reduced sperm production often leads to infertility, as the sperm may not meet the criteria for normal morphology and progressive motility [26]. Research indicates that females experience the highest rates of infertility. Women’s educational level, age at marriage, abortion rates, alcohol consumption, pre-existing medical conditions, and body mass index significantly correlate with infertility. Similarly, men’s characteristics, including their job status, addiction behaviors, smoking, and the presence of underlying health issues, are also significantly linked to these infertility factors [27]. In the context of infertility, studies indicate that around half of the cases are related to problems on the female side. Male factors contribute to about 20 to 30% of infertility cases, while the remaining 20 to 30% result from a combination of issues in both men and women [24]. The primary factors contributing to infertility include advanced maternal age, damage to the fallopian tubes resulting from infections, polycystic ovary syndrome, and endometriosis. Nevertheless, any condition that adversely affects the anatomy of the reproductive organs, ovarian reserve, or the hypothalamic-pituitary-ovarian axis may lead to infertility. In recent years, researchers and health organizations have increasingly recognized male infertility as a pressing public health challenge worldwide. Research conducted over the past few decades has shed light on the complex origins of this condition. Congenital reproductive disorders, genetic alterations, and endocrine or metabolic dysfunctions contribute to male infertility and subfertility. Moreover, external factors such as exposure to environmental pollutants and lifestyle-related problems, including the illegal use of psychoactive drugs, have been demonstrated to have detrimental effects on spermatogenesis [28]. Other common factors that can lead to infertility are genetic disorders like Turner syndrome, Klinefelter syndrome, fragile X syndrome, and cystic fibrosis. Additionally, changes in the androgen receptor, a lack of the azoospermia factor c segment on the Y chromosome, and an expanded CAG triplet repeat within the androgen receptor can also contribute to infertility issues [29, 30].
The perception of childbearing as a vital and natural component of married life creates considerable pressure for couples struggling with infertility, often exacerbated by the expectations of family and community. The challenges associated with infertility can detrimentally influence multiple facets of life for these couples, such as their marital relationship, sexual satisfaction, and psychological well-being. Empirical studies suggest that women facing infertility experience more significant stress regarding their condition than men do. The prevailing cultural belief associates infertility with a significant failure of women to meet their expected roles, explaining this phenomenon. Many women facing infertility often believe that the duty to produce offspring and sustain the family heritage is primarily theirs. Consequently, they may struggle with feelings of inadequacy in their womanhood when they are unable to have children [31].
Overall, infertility is a significant public health issue affecting both men and women worldwide. It is categorized into primary and secondary types based on previous pregnancy history. Female infertility often arises from menstrual and ovulation disorders, uterine issues, and conditions such as polycystic ovary syndrome and endometriosis. On the other hand, factors such as decreased sperm production, quality issues, congenital abnormalities, genetic variations, and lifestyle choices contribute to male infertility. Social pressures and cultural expectations heighten the stress experienced by infertile couples, particularly women.
The role of tissue engineering and regeneration medicine in addressing infertility
Despite the presence of numerous traditional methods like hormonal and surgical interventions, there is still an absence of effective treatments available for individuals afflicted with severe reproductive system disorders, encompassing congenital and acquired abnormalities, traumatic incidents, malignant tumors, inflammation, infectious causes, and iatrogenic damage. The field of tissue engineering shows potential for advancing reproductive medicine by introducing biological substitutes [32].
Understanding the interactions between native tissues and the endocrine system is crucial for developing engineered technologies for both the male and female reproductive tracts. The female mammal reproductive system includes the ovaries, cervix, uterus, fallopian tubes (known as oviducts in non-primate species), and vagina. The processes of sexual intercourse, fertilization, implantation, and the sustenance of pregnancy until term are contingent upon the intricate physiological interactions among the organs of the reproductive tract, which operate by hormonal directives emanating from the ovaries, the pituitary gland, and the hypothalamus during the ovulatory cycle. The principal constituents of the male mammalian reproductive tract encompass the testes, penis, seminal vesicle, epididymis, vas deferens, prostate gland and. Unlike the cyclical nature of female ovulation, the reproductive endocrine cycle in males is distinguished by a daily fluctuation in testosterone levels, predominantly synthesized by the Leydig cells within the testes, in response to signals from the pituitary and hypothalamic regions [33, 34].
Reproductive tissue engineering (REPROTEN) focuses on the reproductive system in regenerative medicine. This capacity holds great promise as a method to enhance fertility and the well-being of individuals with reproductive disorders through the creation, substitution, or regeneration of cells, tissues, and organs within the urinary, sexual, and reproductive systems [35].
A century of innovation in reproductive tissue and organ transplantation, along with advancements in reproductive biology, materials science, bioengineering, and advanced manufacturing, has led to significant progress in REPROTEN, evolving from the initial motivations to advanced technologies such as 3D bioprinting, microfluidics, organoids, and sophisticated bioreactors [36–50]. In this manner, innovative biomaterials enable the creation of complex biomimetic constructs, offering extensive opportunities for engineering living reproductive tissues and organs by integrating diverse cellular sources and differentiation stages. Biomaterials used in reproductive tissue engineering must match the specific mechanical properties, degradation rates, structure, functionality, and physiological conditions of different organs and tissues [51]. The testis and ovary possess complex structures essential for germ cell and oocyte production. The physiological complexities of follicular development, spermatogenesis, and ovulation require interactions among stratified epithelium, gametogenic cells, and fluctuating sex hormone levels [47, 52, 53]. This necessitates specific biomaterials to replicate the microenvironments of the testis and ovary. These biomaterials typically need a microporous structure to facilitate cell interaction, low internal pressure and mechanical rigidity, and a hydrophilic environment for effective mass transfer of cytokines and hormones [54, 55]. Biomaterials for in-situ regeneration offer strong mechanical support, boost vascular regeneration, and reduce inflammation [56, 57].
In addressing female infertility and related reproductive issues, TERM offers excellent potential. It employs biomaterials, cells, and growth factors to enhance the body’s natural regenerative abilities for repairing reproductive organs [58]. These technologies can address reproductive organs issues such as ovarian and uterus failure, as well as issues related to sexual and urinary organs. In females, the ovaries are vital for germ cell development and steroid sex hormone production, but they can be affected by benign and malignant conditions that disrupt their functions. Researchers and experts are actively developing various strategies to improve hormone production and enhance fertility for those affected by these issues. They have investigated a cell-based implant strategy to address the limitations of hormone replacement therapy in individuals suffering from premature ovarian insufficiency, aiming to incorporate cells capable of interacting with the hypothalamic axis to improve hormone secretion patterns and restore ovarian function [35, 59]. These investigations highlight the potential of cell-based implants mimicking ovarian follicles in reducing the side effects of hormone replacement therapy. Collagen, the predominant protein found within the ovarian environment, was the primary natural polymer that underwent examination in the context of encapsulating mouse follicles [60]. However, the investigation into more suitable alternatives has been continued because of the identified limitations [61–63].
Uterine issues are another significant factor in female infertility, highlighting the need for new treatment solutions. The uterus has two main layers: the endometrium, which helps with the implantation of embryos, and the myometrium. Creating a bioengineered uterus is challenging due to its unique structure and functions. A bioengineered uterus could facilitate organ reconstitution in specific clinical settings [64, 65]. In this context, substantial advancements have been attained in bioengineering the uterus, including the development of endometrial organoids [66], decellularized uterine scaffold [67, 68], and Uterine and Oviduct tissue engineering using stem cells with various cell sources [69–71]. Additionally, in other reproductive organs of women, TERM methods have been employed to help individuals with cervical pathologies, such as cervical dysfunction and cancer [35]. In this regard, various studies exploring the engineering of sexual and urinary organs have been conducted [72–74].
On the other hand, cancer treatments such as chemotherapy and radiotherapy can harm the testicles, potentially resulting in infertility [75]. Researchers have found that decellularized testicular tissue creates a favorable setting for stem cells to develop into specific testicular cell types [76]. Similarly to the progress made in the engineering of ovaries, a range of hydrogels have also been used to encase testicular cells and create in vitro representations of the testis [77, 78]. Another novel strategy has been used in vas deferens tissue engineering, yielding favorable outcomes [79, 80]. Significant progress in the improvement of male infertility may be realized, particularly in cases of obstructive azoospermia linked to the congenital absence of bilateral vas deferens, which is present in approximately 1% of men facing infertility challenges [81]. Additionally, conditions like cancer, trauma, or both congenital and non-congenital disorders affecting men’s sexual and urinary organs, particularly the penis, can result in problems with function or even the loss of the organ. The solution entails surgical intervention to repair or substitute the affected tissues. Despite their potential, current regenerative methods are limited by challenges related to tissue compatibility and availability. Researchers and medical professionals have commenced investigating the principles of regenerative medicine and tissue engineering to revive and repair the genitourinary system like rebuilding corporal bodies and urethral, targeting disorders such as epispadias, ambiguous genitalia, micropenis, hypospadias, and others. The project started with the goal of creating an artificial penis made from bone cartilage to address injuries caused by trauma [82]. Erectile dysfunction (ED) is another disorder that impacts the reproductive capacity of males. ED is a common condition where a person can’t attain or preserve an erection for acceptable sexual action. ED is a widespread issue that affects over half of men aged 40 to 70, potentially leading to severe impacts on their quality of life. Physiological, hormonal, vascular, and neurological factors usually cause it [83]. In this context, positive outcomes have been realized in the implementation of tissue engineering in animal models, showing great potential for upcoming utilization in human patients [84–86]. For example, stem cell therapy (SCT) shows promise as a treatment for ED, demonstrating positive results in both animal studies and human cases. Essential developments in stem cell research include induced pluripotent stem cells (iPS) and embryonic stem cells (ES). However, the practical use of these cells in clinical settings remains limited due to concerns about safety and the possibility of uncontrolled cell growth leading to tumors or abnormal tissue formation [87].
Overall, the advancements in REPROTEN have led to the development of biomimetic constructs that enable the creation of sophisticated reproductive tissues and organs, thus providing opportunities to repair reproductive abnormalities. However, despite these improvements, the challenges remain. A deeper understanding of reproductive tissue microenvironments is essential for replicating native conditions. Selecting appropriate cell sources, ensuring biocompatibility, and achieving scaffold neovascularization are critical. Ethical, regulatory, cost, commercialization, and insurance coverage issues also need consideration. Ethical considerations of REPROTEN in humans require thorough addressing, focusing on the safe translation of animal findings, the efficacy of products, and patient rights. Considerations should include consent, therapy access, patient monitoring, and potential adverse effects. So, stakeholders need to create ethical guidelines as the field of REPROTEN continues to develop. Also, developing these frameworks must demonstrate an understanding of engineering benefits and a commitment to patient safety, individual autonomy, and societal health. Additionally, tackling ethical dilemmas in REPROTEN may lead to substantial transformations in medical therapies and greatly affect patient management [35].
Hydrogels and their properties
Hydrogels are complex three-dimensional (3D) viscoelastic polymer nanostructure systems that do not dissolve in water. Hydrophilic polymers form these structures by creating specific chemical or physical crosslinks. The presence of hydrophilic functional groups, such as hydroxy, carboxyl, amine, and sulfate, facilitates this process. Hydrogels can absorb a large amount of water and expand without breaking apart, similar to natural tissues and the extracellular matrix (ECM) [88]. This makes them potentially useful for biological applications. The presence of ionic interaction and hydrogen bonding in the structure of hydrogels prevents them from dissolving in water. It helps maintain the necessary mechanical strength and physical integrity of polymer hydrogels [89]. Hydrogels, classified based on their raw materials, fall into two main categories: synthetic and natural. Natural hydrogels are particularly valuable because of their high biocompatibility and structural similarity to the ECM. However, they sometimes have limitations, such as low mechanical strength and rapid degradation. In contrast, synthetic hydrogels offer a controllable 3D environment with tunable mechanical properties and degradation rates. However, they may need to catch up to natural hydrogels regarding bioactivity [88]. Researchers have explored hybrid approaches by merging natural and synthetic polymers to address these challenges. This strategy allows for creating functional hydrogels that harness the best of both enhanced mechanical properties from synthetic components and biological relevance from natural ones [90].
Owing to their exceptional properties, hydrogels have emerged as compelling candidates for various medical applications, including infertility treatment. Hydrogels possess remarkable properties, such as water absorption, softness, ECM. So, hydrogels are emerging as promising technologies for drug delivery, gene therapy, tissue engineering, and wound dressing. Interestingly, the versatility of hydrogels extends to applications like contact lenses and tuberculosis treatment [91]. Hydrogels can be classified into different categories based on various criteria. For instance, the classification may consider their crosslinking methods or the type of structure they possess, such as crystalline, amorphous, or supramolecular. Additionally, the nature of their side groups can be examined to determine whether they are ionic or nonionic. Their structural characteristics also come into play, as hydrogels may be homo-polymers or copolymers. Furthermore, assessing their physical properties focuses on whether they are crystalline, amorphous, or supramolecular. Finally, their responses to external factors like light, temperature, electromagnetic radiation, pH, and ultrasound can also form a basis for classification [92].
Selecting an appropriate hydrogel for infertility treatment involves careful consideration of the mechanical and physicochemical properties specific to the target tissue. Ideally, bioactive hydrogels exhibit desirable properties, including biocompatibility, adjustable mechanical effects, and the capacity to release therapeutic agents sustainably [93]. These hydrogels create an environment conducive to encapsulating and transporting cells, supporting critical biological processes such as angiogenesis and cell absorption. Hydrogels have a wide range of uses based on the type of damage and the reasons behind infertility. They can be utilized in fields such as tissue engineering, medication delivery, and cell therapy.
Effective polysaccharides used in tissue engineering for treating infertility
Polysaccharides such as alginate and hyaluronic acid (HA) are used in tissue engineering for infertility treatments because of their natural degradation, compatibility with biological systems, and ability to form hydrogels. These polysaccharides form suitable environments for cell growth and tissue regeneration. They play a specific role in fertility treatments, such as encapsulating ovarian tissue, stimulating stem cell delivery, promoting tissue repair, and supporting endometrial regeneration and folliculogenesis [94, 95]. Other polysaccharides, including chitosan and heparin-based hydrogels, enhance cell adhesion and promote angiogenesis [96]. Modified versions of polysaccharides, such as dextran and cellulose derivatives, have enhanced mechanical stability and bioactivity for tissue engineering applications [97, 98]. These polysaccharides offer promising therapeutic solutions for infertility issues and are expanding in use for reproductive tissue engineering. Table 1 provides an overview of various polysaccharides, highlighting their biochemical properties and advantages that make them promising candidates for applications in tissue engineering and potential use in infertility treatment.
Table 1.
Summary of biochemical properties, advantages, and challenges of polysaccharides for tissue engineering and regenerative medicine
| Polysaccharide | Properties/ Advantages | Challenges | References |
|---|---|---|---|
| Sodium Alginate |
Forms stable hydrogels upon ionic crosslinking (e.g., calcium ions), Biocompatible, Biodegradable, Non-toxic |
Modifications are needed for specific applications, Improvements in cell adhesion are required |
[99, 100] |
| Hyaluronic Acid (HA) |
Naturally presents in the extracellular matrix, Hydrophilic, Forms hydrogels through chemical crosslinking |
Rapid degradation occurs in vivo, Requires chemical crosslinking for stability |
[101, 102] |
| Chitosan |
Forms hydrogels in acidic conditions, Biocompatible, Supports cell adhesion, Biodegradable, Exhibits antimicrobial properties |
Exhibits limited solubility at physiological pH, Requires functionalization for enhanced efficacy |
[103] |
| Dextran |
Hydrophilic, Forms hydrogels when chemically modified, Biocompatible |
Rapid removal from the body, Requires structural modifications to maintain efficacy |
[104, 105] |
| Heparin |
Often used in combination with other polysaccharides, Promotes growth factor stabilization, Biocompatible, Easily modifiable |
Potential immunogenicity, Rapid degradation |
[106–108] |
| Cellulose Derivatives |
Biocompatible, Easily modifiable |
Exhibits limited biological activity, Needs to be combined with other biomaterials to enhance cell adhesion |
[109, 110] |
| kappa-Carrageenan |
Forms gels, Exhibits high biocompatibility, antitumor, antioxidants immunomodulatory, and anticoagulant properties |
Shows variable rates of degradation, Potential to trigger an immune response |
[111, 112] |
| Pullulan |
Water-soluble, Non-toxic, Biodegradable |
Mechanical properties need improvement for enhanced performance | [113] |
| Agarose |
Thermo-reversible gelation, Biocompatible |
Exhibits limited biological activity, Must be combined with other materials for effectiveness |
[114–116] |
| Pectin |
Biodegradable, Non-toxic |
Exhibits poor mechanical strength, Requires chemical modification for effectiveness |
[117, 118] |
| Starch |
Biocompatible, Biodegradable, Easily available, Low-cost |
Exhibits poor mechanical strength, Requires modification for improved bioactivity |
[119, 120] |
Alginate
Alginate is a linear polysaccharide from a natural source extracted from brown seaweed and bacteria such as Azotobacter and Pseudomonas. It consists of 1, 4 (-D-mannuronic acid) (M) and (-L-guluronic acid) (G) homo-polymeric units. Alginates are obtained from various sources containing different M and G, affecting the material’s properties [121]. Alginate has attracted attention because of its outstanding possessions such as non-toxicity, biocompatibility, biodegradability, easy processing, gel-forming ability, high availability, and low cost in regenerative medicine applications and infertility treatment [122].
Hyaluronan
Hyaluronic acid, also known as hyaluronan, is a type of carbohydrate called glycosaminoglycan (GAG). It consists of a series of repeating disaccharide units comprising N-acetyl glucosamine and D-glucuronic acid. This polymer is abundantly found in the ECM and mammalian cells [123]. The human body produces a significant amount of HA, crucial in various cellular interactions, including fertilization, proliferation, development, and molecular recognition. HA also contributes to some physiological functions such as lubrication, maintaining hydration balance, structural support in ECM, and steric interactions [124]. Hyaluronic acid hydrogel is particularly valuable in regenerative medicine and infertility treatment because of its high water absorption and excellent tissue compatibility [125].
Chitosan
Chitosan results from the deacetylation of chitin, a naturally insoluble substance that comes from fungi and the exoskeletons of arthropods, such as insects and crustaceans. This polymer has received significant interest because of its biodegradability and biocompatibility [13]. It also has low toxicity and immunostimulant activities. In tissue engineering, converting chitin to chitosan increases the number of amino groups and enhances its water solubility. Chitosan is unique as it is the only natural cationic polymer, possessing reactive amino groups [13]. Consequently, researchers widely utilize it to produce hydrogels for various pharmaceutical applications, tissue engineering, and infertility treatments [126].
Heparin
Heparin is a linear polysaccharide that serves as a blood anticoagulant by binding to anti-thrombin, a serine protease inhibitor. Researchers primarily extract it from natural tissues like pig intestines and cow lungs, which display considerable heterogeneity in their chemical structures and molecular weights [127]. Heparin is involved in numerous biological processes by interactions with diverse proteins and hydrogels [108]. Compounds containing heparin display attractive properties, including growth factor binding, anticoagulant activity, and anti-apoptotic effects, making them promising candidates for new applications in infertility treatments [107].
Applications of polysaccharide-based hydrogels in infertility treatment
Tissue engineering, which combines biology and engineering to create viable substitutes for damaged tissues, is becoming a promising solution in reproductive medicine. This approach addresses the limitations often seen with conventional methods [128]. Reproductive tissues are intricate organs that have unique roles in the reproductive process. While these tissues are quite complex, recent progress in tissue engineering has made it feasible to repair and regenerate them using biological materials [62]. Researchers propose using natural bio-polysaccharide materials to regenerate reproductive organs, as these materials can replicate key characteristics of the natural ECM. They also encourage important cell activities like sticking to surfaces, moving around, developing into specific cell types, and producing ECM. They are also highly biodegradable and compatible with living tissue [16]. Besides their applications in tissue engineering, these hydrogels can create a suitable environment for encapsulating and transferring cells and testicular/ovarian tissues, while promoting essential biological processes like angiogenesis and cell absorption. For example, alginate hydrogels are great at mimicking the natural extracellular matrix. They can release important bioactive molecules that help with new blood vessel formation and healing in ovarian and testicular tissues [94, 95]. Hyaluronic acid hydrogels are also beneficial; they promote cell growth and differentiation by interacting with CD44 and RHAMM receptors on both epithelial and stromal cells, supporting regeneration of the endometrium [129–131]. On the other hand, chitosan derivatives stand out for their antimicrobial and biocompatible qualities. They help create a nurturing environment for follicular maturation by managing immune responses and improving cell interactions and their surrounding matrix [132]. These properties allow polysaccharide hydrogels to repair damaged reproductive tissues effectively, aid in cellular regeneration, and help restore reproductive functions. Detailed applications of polysaccharides in infertility treatment are elaborated in Table 2, along with their specific mechanisms and targeted conditions (Table 2).
Table 2.
Detailed applications of Polysaccharide-Based hydrogels in infertility treatment
| Polysaccharide | Applications in Infertility Treatments | Mechanisms/Functions | Targeted Conditions | References |
|---|---|---|---|---|
| Sodium Alginate |
Ovarian tissue encapsulation, Delivery of stem cells for ovarian repair, Follicle culture, 3D Culture system for testicular/ovarian tissues, Controlled delivery of growth factors |
Easily forms hydrogels, Supports cell encapsulation, Reduces ischemic damage, Possesses mucoadhesive properties for targeted drug delivery, Promotes angiogenesis and tissue repair |
Ovarian tissue transplantation, Premature ovarian failure, Ovarian and testicular regeneration |
[43, 94, 133] |
| Hyaluronic Acid (HA) |
Endometrial regeneration, Follicular maturation, Stem cell delivery |
Supports cell migration, proliferation, and angiogenesis, Provides structural support for stem cells |
Thin endometrium, Asherman’s syndrome, Endometrial repair, Embryo implantation |
[124, 131, 134] |
| Chitosan |
Follicular maturation, Endometrial repair |
Enhances cell adhesion, Provides a supportive microenvironment, Easily modifiable |
Follicle culture, Oocyte maturation, Damaged endometrial tissue |
[96, 135, 136] |
| Dextran |
3D culture systems for testicular/ovarian tissues, Follicular maturation, Ovarian tissue engineering, Drug delivery for ovarian repair |
Stabilizes growth factors, Easily modifiable, Supports follicle maturation |
Ovarian tissue culture | [2, 97, 137] |
| Heparin | Controlled release of growth factors |
Stabilizes bioactive agents, Promotes angiogenesis, Prevents clotting, Enhances cell signaling |
Endometrial regeneration, Uterine repair |
[20–22] |
| Cellulose Derivatives |
Scaffold for ovarian cell culture, Supporting tissue regeneration |
Provides structural stability, Provides scaffolds for cell culture, Prevents tissue adhesion |
Ovarian tissue repair, Adhesion prevention |
[138–141] |
| kappa-Carrageenan | Ovarian tissue scaffolding and matrix |
Cross-linkable with other materials, Forms hydrogels, Supports stem cell delivery |
Ovarian tissue engineering | [142–144] |
| Pullulan |
Endometrial tissue regeneration, Stem cell-based therapies, |
Promotes cell attachment and proliferation, Prevents tissue adhesion |
Endometrial repair, Adhesion prevention |
[145–147] |
| Agarose | 3D culture systems for ovarian follicles and stem cells |
Supports in vitro 3D cell culture, Provides structural support |
In vitro fertilization | [2, 148–151] |
| Pectin |
Scaffold for ovarian cell delivery, Tissue repair applications |
Forms biodegradable hydrogels, Encourages cell encapsulation |
Ovarian tissue repair | [152, 153] |
| Starch | Ovarian tissue scaffolding and drug delivery | Encapsulates cells and drugs | Ovarian tissue regeneration | [154, 155] |
Therefore, polysaccharide-based hydrogels have various applications, ranging from tissue engineering to drug delivery and cell therapy, depending on the type of damage involved (Fig. 1). The following sections explore the applications of polysaccharide-based hydrogels in regenerative medicine, specifically focusing on their roles in addressing reproductive system-related conditions.
Fig. 1.
Polysaccharide-based hydrogels in infertility treatment of men (a-b) and women (c-f). (a) Hydrogels encapsulated with ITT and VEGF, (b) Culturing of testicular tissue encapsulated in hydrogels, (c) ApoBDs-loaded hyaluronic acid hydrogel, d) Culturing of hydrogels encapsulated with stem cells, follicles, ovaries, and drug, (e) 3D printing of hydrogels containing iMSCs, (f) 3D printing of hydrogels containing EEC and ESC. ITT: immature testicular tissue, VEGF: vascular endothelial growth factor, ApoBD: apoptotic bodies derived from mesenchymal stem cells, EEC: endometrial epithelial cells, ESC: endometrial stromal cells, iMSCs: induced pluripotent stem cells. Created by the authors using CorelDRAW
Enhancing oocyte maturation with polysaccharide-based hydrogels
The ovaries, the female reproductive organs, produce sex hormones such as estrogen and progesterone. They are also crucial for the growth and development of oocytes. Hence, any disturbance in their regular function may result in infertility. Premature ovarian failure (POF) happens when the ovaries stop functioning normally before the age of 40 [156]. Therefore, it can meaningfully reduce the quality of life of affected people. POF can be a side effect of chemotherapy, posing a significant concern for young women with cancer. It is commonly related to abnormal sexual hormones, ovulatory dysfunction, and infertility, making it essential to develop effective treatments for this condition [157]. For effective treatment, regenerative medicine has received much attention as one of the promising methods in recent years [158–160]. One approach in this area involves directly injecting mesenchymal stem cells (MSCs) into the ovary to treat premature ovarian failure (POF) [161–163]. Although this technique enhances ovarian function and fertility in rodent studies, it does face challenges regarding cell survival [164, 165]. Recent suggestions propose that using hydrogels can overcome these issues [166]. Additionally, transferring cells to the ovary using hydrogels allows for the concentration of cells within the hydrogel, minimizing their spread to other organs [167]. Research indicates that this approach shows potential for boosting clinical pregnancy rates in women with primary ovarian failure following transplantation [168]. In general, there are two common strategies for using hydrogels. Researchers use them as carriers for follicles to treat POF or as an ECM to culture human and murine follicles in vitro [94, 169, 170].
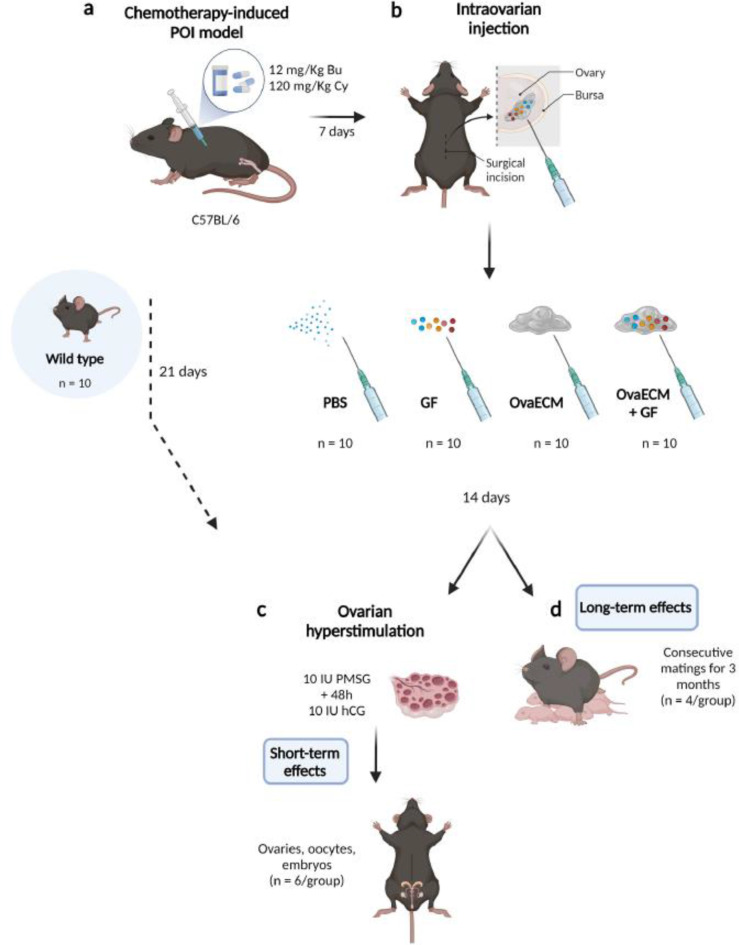
In one of the in vitro approaches, researchers use hydrogel coatings in two-dimensional cell culture to enhance the behavior of cells on culture plates. By covering the plates, they promote better cell adhesion and proliferation. These special coatings often consist of specific types of proteins and natural tissue matrices that can create particular cellular interactions [171–173]. A research study by Francés-Herrero et al. [173] investigates the use of decellularized bovine ovarian cortex extracellular matrix hydrogels (referred to as OvaECM), both with and without added growth factors (OvaECM + GF), in a murine model designed to mimic chemotherapy-induced premature ovarian insufficiency (POI) [173] (Fig. 2). The OvaECM + GF treatment revitalized the process of follicle development, improved the ovarian environment, and reduced cell death. Treated mice showed better reproductive outcomes and normal function after two breeding cycles, highlighting OvaECM’s potential as a treatment for POI [173].
Fig. 2.
POI model generation and therapeutic methods, including chemotherapy (a), intraovarian injections of PBS, GF, OvaECM, or OvaECM + GF (b), and ovarian repair and fertility outcomes evaluation (c and d). The abbreviations include GF for growth factor, hCG for human chorionic gonadotropin, OvaECM for bovine ovarian cortex extracellular matrix, and PMSG for pregnant mare serum gonadotropin. Reprinted with permission from Ref [173], Copyright (2025), open access article distributed under the terms and conditions of the Creative Commons CC-BY-NC-ND license
However, to overcome some limitations, such as lower rigidity compared to native ovaries, there is a growing tendency to use hybrid hydrogels based on polysaccharides, especially alginate [168, 174]. In a research study that utilized alginate and decellularized ovarian extracellular matrix (OvaECM), hybrid hydrogels were developed with the right texture to enable the growth of follicles [175] (Fig. 3). In this study, higher concentrations of alginate led to a corresponding rise in the rate of follicle recovery. However, due to some limitations, especially low degradability, pure alginate is unsuitable for use in the ovary, and it is better to use it as a hybrid [175].
Fig. 3.
(a) Creation of a hydrogel based on decellularized oECM from bovine ovaries through mechanical dissociation, enzymatic and detergent treatments, lyophilization, and pepsin digestion, designed for follicle encapsulation and in vitro culture. (b) Effect of oECM2-alginate hydrogel mixtures on human ovarian follicle morphology after 7 days in vitro. (i) 90% oECM2 + 10% alginate, (ii) 75% oECM2 + 25% alginate, (iii) 100% alginate. Black arrows show human follicles. The transparency and homogeneity of hydrogel beads increased with higher alginate content. Reprinted from Ref [175], open access article distributed under the terms and conditions of the Creative Commons CC BY license
Besides, in the area of follicle maturation, hydrogels can be engineered to create a cellular environment that promotes the maturation of ovarian follicles. In this context, tissue engineering has made it possible to reconstruct a 3D structure of the ECM for follicle development. Since polysaccharide-based hydrogels have created an environment similar to in vivo conditions for successful maturation in vitro, they are currently promising in supporting oocyte maturation in vitro [176]. Hydrogels provide the necessary physical and biochemical cues for optimal oocyte development and offer a potential solution for couples facing female infertility problems [177]. Traditional 2D cell cultures cannot replicate the natural cell environment. Therefore, using hydrogels for 3D cultures provides a better way to simulate how cells interact, grow, and develop under conditions similar to those found in living tissues [178, 179]. Rios et al. [174] investigated using alginate hydrogels to encapsulate ovarian follicles, allowing for maturation after transplantation to a different location in the body [174]. The study demonstrated the survival and growth of multiple follicle populations and successful fertilization of collected oocytes. The hydrogels also stopped metastatic breast cancer cells from spreading when they were transplanted together. This innovation could offer new pathways for young female cancer patients to protect their fertility [174].
Similarly, chitosan is recognized for its high biocompatibility and adjustable mechanical properties, positively influencing follicle development. In a research study, Khunmanee et al. [132] generated a three-dimensional microenvironment for the in vitro culture of mouse preantral follicles. They combined thiolate hyaluronic acid (HASH) with thiolate chitosan (CSH) and alkylated β-cyclodextrins (Alkyl-β-CD) to create chitosan-thiolate hyaluronic hydrogel (CSHS) [132] (Fig. 4). The purpose of designing these biomaterials was to facilitate nutrition, maintain structural integrity, secrete and transfer hormones, and improve the quality of mature eggs, and improve meiosis spindle formation. To evaluate the hydrogels, each follicle was encapsulated in a hydrogel for 10 days, with mature eggs ovulated on the 11th day. The findings indicated that, in addition to promoting follicle growth in vitro and facilitating oocyte maturation, the CSHS hydrogel supports the survival of follicles and the viability of mature oocyte cells, exhibiting the typical characteristics of the meiotic spindle, chromosomal alignment, and maintaining a spherical morphology. These results demonstrate that the CSHS hydrogel benefits follicle growth and survival [132].
Fig. 4.
A schematic design illustrating the morphology of ovarian follicles at various stages, along with the process of isolating these follicles from a mouse ovary, and encapsulating them in a hydrogel made from thiolated chitosan and thiolated hyaluronic acid. Reprinted with permission from Ref [132], Copyright (2025), open access article distributed under the terms and conditions of the Creative Commons CC-BY-NC-ND license
Researchers have also modified polysaccharide hydrogels with a peptide mimicking the extracellular matrix (ECM). This modification promotes an ideal environment for follicle growth and actively influences the behavior of human granulosa cells (hGCs) [180]. This strategy ultimately leads to the formation of organoids, which are self-assembled three-dimensional multicellular structures in vitro. These constructs mimic their parent organ better than standard 2D cell cultures. Zhao and colleagues found that 3D culture systems significantly enhanced the survival, growth, and viability of follicles, while also improving the meiotic competence of oocytes [180]. Moreover, human granulosa cells grown in a 3D culture revealed slower aging, lower levels of oxidative stress, and higher mitochondrial membrane potential. This suggests that this type of environment could effectively support the development of follicles [180]. Since artificial ovary (AO) using alginate hydrogels has limitations in supplying hormone levels to post-menopausal women, researchers have created chitin-based (CTP) hydrogels, which support cell growth and blood vessel formation [96]. In experiments, follicles in CTP hydrogels showed better development and hormone production than alginate hydrogels. Transplantation of follicles in CTP hydrogels into mice displayed enhanced hormone levels and prevented bone loss and reproductive organ atrophy. The outcomes demonstrated the potential of using AO created with CTP hydrogels to treat menopausal symptoms [96].
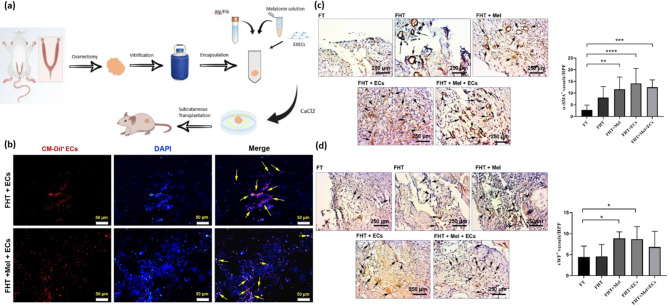
One more factor contributing to infertility is the ischemic niche, which can lead to the degeneration of follicles after transplanting cryopreserved and thawed ovaries to different locations in the body. Transplanting frozen immature testicular or ovarian tissue is a valuable option for enabling young boys and girls who have undergone chemotherapy and radiation therapy for cancer in childhood to have the possibility of becoming parents in the future. In such situations, freezing undeveloped tissue is the only option to safeguard future fertility [43, 160]. The results of the studies showed that in ovarian tissue transplants, revascularization and oxygenation occur within 5 days, which leads to tissue hypoxia. As a result, ischemic stress causes tissue apoptosis or necrosis [181]. Enhancing blood flow is an effective way to minimize ischemic damage to ovarian follicles. Research on ovarian and testicular tissue transplantation has investigated strategies to mitigate ischemic damage. Studies have focused on using antioxidants and growth factors known for their effectiveness in facilitating the development of blood vessels [70]. A study investigated a hydrogel composed of alginate and fibrin, enriched with Melatonin and endothelial cells, to enhance blood vessel formation in cryopreserved and thawed ovaries transplanted in rats [94]. In this study, Izadpanah and colleagues focused on evaluating how well a hydrogel composed of fibrin (Fib) and alginate (Alg), infused with Melatonin (Mel) and endothelial cells (ECs), promotes the growth of new blood vessels [94]. They used this hydrogel to encase cryopreserved and thawed ovaries before transplanting them to different locations in rats (Fig. 5) [94]. The results showed that the interaction between alginate and fibrin was successful, and the hydrogel containing Alg + Fib had a higher rate of biodegradation and swelling than the alginate group. Also, the survival of encapsulated EC cells increased, and the number of blood vessels improved in the presence of Mel and ECs. This study showed that simultaneous use of Alg + Fib with ECs and Mel stimulated angiogenesis and reduced fibrotic changes in encapsulated cryopreserved/thawed ovarian transplants [94].
Fig. 5.
(a) Schematic illustration of experimental procedure. (b) Tracking of Red CM-Dil labeled CD144 + endothelial cells 14 days post-transplant in a rat model. Red ECs are observed in the FHT + ECs and FHT + Mel + ECs groups (yellow arrows). Nuclei are stained with DAPI and appear blue. (c-d) IHC staining assessed vascular density (n = 10). The number of α-SMA+ vessels enhanced in hydrogels with Mel, ECs, and Mel + ECs compared to FHT and FT groups (Black arrows; c). Similarly, vWF+ vessels increased in FHT + Mel and FHT ECs groups against FT (Black arrows; d). Overview of the experimental groups, including a control group, rats that received naked vitrified/thawed ovaries (FT), rats transplanted with Alg-Fib hydrogel containing vitrified/thawed ovaries (FHT), and additional groups receiving the hydrogel with either Melatonin (FHT + Mel), endothelial cells (FHT + ECs), or a combination of both Melatonin and endothelial cells (FHT + Mel + ECs). One-way ANOVA with Tukey post hoc analysis. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. Reprinted from Ref [94], open access article distributed under the terms and conditions of the Creative Commons CC BY license
Researchers are also utilizing HA-based hydrogels to mitigate tissue ischemic damage. Rapid rebuilding of blood vessels is essential to minimize ischemic injury following ovarian transplantation [182]. In mice, revascularization after ovarian transplantation typically occurs within 48–72 h [183]. Therefore, it is crucial to investigate new approaches to accelerate transplant revascularization and reduce post-transplantation hypoxia. HA is a vital element of the ECM and is instrumental in initiating and regulating the process of angiogenesis [184]. On the other, research indicates that the fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF) play a vital role in angiogenesis within the ovary [185]. In this context, Tavana et al. [130] assessed the impact of a hyaluronic acid hydrogel scaffold (HABH) on newly transplanted rat ovarian tissue, analyzing its effects both with and without the presence of VEGF and bFGF [130]. They assigned twenty-four rats to three groups: Group A consisted of mice with ovarian tissue that lacked HABH, VEGF, and bFGF; Group B included mice with ovarian tissue enclosed in HABH; and Group C comprised mice with ovarian tissue enclosed in HABH, which contained VEGF and bFGF. After three days, researchers conducted histological and hormonal analyses to assess the number of follicles, hormone levels, and the expression of genes associated with angiogenesis and apoptosis. The findings suggested that encapsulating the ovaries with HABH, even without growth factors, can help preserve follicles by reducing ischemia, promoting angiogenesis, and restoring hormone levels [130].
Other research has also provided a novel strategy for delivering human amniotic epithelial cells (hAECs) to treat POF [186]. A recent study found that hAECs have a remarkable ability to help improve ovarian function in women experiencing premature ovarian failures due to chemotherapy [186]. Researchers explored the possible therapeutic benefits of hAECs or the conditioned medium (CM) derived from these cells, entrenched in a composite hydrogel made from sodium alginate and bioglass (SA-BG). The study was conducted on mice that suffered from chemotherapy-induced POF. The findings indicated that transplanting human amniotic epithelial cells and conditioned medium encapsulated in SA-BG aided restore follicle maturation, enhanced granulosa cell function, and boosted ovarian blood vessel formation in mice with premature ovarian failure [186].
Uterine tissue reconstruction using polysaccharide-based hydrogels
Intrauterine adhesions, often known as Asherman’s syndrome, can occur due to trauma and may block the uterine cavity either partially or completely. This can lead to difficulties in getting pregnant or repeated miscarriages [187]. While physical barrier treatments and drug or cell therapies are effective methods for addressing intrauterine adhesions (IUAs), they have limitations like increased adhesion, immunogenicity, and fibrosis. Recent approaches combining physical barrier treatments with cell or drug therapy using hydrogels offer promising solutions to challenges. One of the innovative ways to overcome these challenges is to use polysaccharide-based hydrogels [188]. These hydrogels possess high water absorption, elasticity, and swelling characteristics, exhibiting properties analogous to soft tissues. These characteristics allow them to affect the uterine microenvironment and make them one of the most suitable materials for treating IUA and endometrial regeneration [5, 189, 190] (Fig. 6). Therefore, there’s a need to create an injectable hydrogel that has strong mechanical properties, good stability, and the ability to break down properly. It should also help prevent fibrosis and promote the growth of endometrial cells [191]. In this regard, sodium alginate plays a significant role in endometrial repair, enhancing the growth and activity of stromal and epithelial cells [192]. Xie et al. conducted a study to address intrauterine adhesions by developing an injectable hydrogel that incorporates platelet-rich plasma (PRP) [193]. This hydrogel was made from a combination of poly (ethylene glycol) diacrylate (PEGDA), sodium alginate (SA), and L-serine. The findings indicated that the hydrogel possesses excellent biocompatibility, favorable degradability, strong mechanical properties, and effective growth factor release capabilities. Both in vitro and in vivo assessments demonstrated its degradability. The hydrogel maintained its stability and integrity for 15 days, after which it gradually decomposed and was naturally eliminated from the uterine cavity within one month. In mouse models of intrauterine adhesions, the in-situ injection of the hydrogel successfully prompted regeneration of endometrial and reduced fibrosis, ultimately improving fertility. This research presented a new approach to treating IUAs and enhancing endometrial regeneration [193].
Fig. 6.
a) An endometrial bilayer structure based on sodium alginate-hyaluronic acid hydrogel (Alg-HA) [194], b) The overall design of preparation of the hiMSC-loaded hydrogel, 3D printing scaffold fabrication, in vitro culture and in vivo transplantation, and assessment of endometrial repair [195], c) Mesenchymal stem cell secretome-loaded HA gel (MSC-Sec/HA gel) intrauterine injection in a rat model [125], d) fabrication of drug delivery system of gelatin methacrylate (GelMA) and sodium alginate scaffold based on a microfluidic droplet template [196], e) The schematic pic of the design and application of HA hydrogel containing apoptotic bodies (ApoBDs) for intrauterine injection to promote endometrial regeneration [197]. Created by the authors using CorelDRAW
Yang et al. [190] also developed an injectable hydrogel based on sodium alginate (SA), polydopamine (PDA), and carboxymethyl chitosan (CMCS) enriched with Arginine (Arg) [190]. This polysaccharide complex hydrogel features double crosslinking facilitated by a multifunctional group. In hydrogels, the degree of fibro tissue and the inflammatory reaction can be reduced by adjusting the absorption of fibrinogen, antioxidants, and antibacterial agents. The effect of hydrogel on endometrial repair in vivo was confirmed through a mouse endometrial injury model. The experimental findings indicated that the SA/PDA/CMCS-Arg hydrogel promotes the growth of endometrial stromal and epithelial cells, encourages gland regeneration, restores the endometrium’s structure, and helps reduce inflammation while preventing fibrosis. In vivo condition, after 7 days of treatment of mouse-damaged endometrium with hydrogel, damaged endometrial epithelium and glands recovered well, and hydrogel was removed. These outcomes showed that the prepared injectable hydrogel has a good possibility of endometrial repair [190].
Besides, In 2023, a study was performed by Nie et al. [194], in which to regenerate the endometrium, an endometrial bilayer (EC) structure based on sodium alginate-hyaluronic acid hydrogel (Alg-HA) was made by 3D extrusion-based bioprinting (EBB) [194]. In this structure, a dense monolayer of endometrial epithelial cells (EECs) was placed in the upper layer, and endometrial stromal cells (ESCs) were arranged in a porous grid-like microstructure in the lower layer [194]. They used this construct in a rat model of uterine damage to repair endometrial morphology and dramatically enhance pregnancy results. The results showed that this two-layer EC structure repaired the structure of the endometrial wall and improved the reproductive results [194].
Moreover, in another study, Ji and colleagues carried out a study where they utilized 3D printing technology to create a hydrogel scaffold infused with human-induced pluripotent stem cells (hiMSCs) [195]. 3D printing ink of sodium alginate and gelatin was used to fabricate this scaffold. The study demonstrated that the 3D printed structure improves the morphology of endometrial tissue in the endometrial injury model and enhances functional indicators of endometrial receptivity. These results suggest that this scaffold is an effective material for endometrial repair [195]. In conclusion, research and the properties of polysaccharide-based hydrogels indicate that incorporating bioactive substances and utilizing 3D printing technology to create bio-scaffolds can significantly enhance the structure and function of the endometrium. This approach shows great potential for advancing infertility treatment strategies.
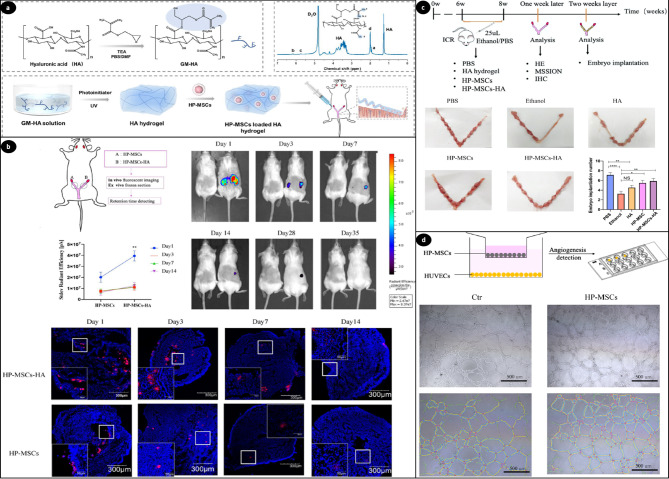
Besides alginate, many researchers have explored the efficacy of hyaluronic acid gel as an effective carrier for intrauterine injection [5]. Numerous studies have investigated the use of HA in infertility therapy. Research indicates that a thin endometrium may lead to infertility, so enhancing its thickness could facilitate pregnancy. On the other hand, treating thin endometrium with mesenchymal stem cells has recently emerged as a promising new strategy. In this regard, HA hydrogel provides structural support to cells by forming a crosslinked three-dimensional network, which can be degraded by endometrium hyaluronidase [198]. Lin et al. introduced a new strategy using HA hydrogel and human placenta-derived mesenchymal stem cells (HP-MSCs) to improve the thickness of the thin endometrium [129]. They hypothesized that this hydrogel improves embryo implantation rates by promoting the migration and proliferation of epithelial and stromal cells and enhancing angiogenesis. The findings indicate that 3D HA hydrogel enhances the survival of HP-MSCs in the uterus and promotes the secretion of growth factors. They investigated the distribution of HP-MSCs using immunofluorescence staining and evaluated the morphological changes in the endometrium through Masson staining, H&E staining, and immunohistochemistry. In addition, examinations conducted in living organisms showed that HP-MSCs paired with HA significantly boosted both the number of glands and the thickness of the endometrium. This research suggests that HA hydrogel is a hopeful option for encapsulating and releasing HP-MSCs for treating patients with thin endometrium (Fig. 7) [129].
Fig. 7.
(a) Schematic of HA hydrogel and HP-MSCs-HA preparation, synthesis process, the 1H-NMR spectrum of GM-HA (glycidyl methacrylate functionalized HA) in D2O, and the instillation of HP-MSCs-HA into a mouse mode. (b) The Retention time of HP-MSCs and HP-MSCs-HA labeled with CM-DiD or CM-DiR, implanted at a density of 2 × 105 cells per uterus. The left mouse represents the untreated control group, while the right mouse represents the treatment group. Retention was assessed at multiple time points: 1, 3, 7, 14, 28, and 35 days. Data were shown as mean ± SEM (n = 3), with ** indicating P < 0.01. The last panel illustrates retained HP-MSCs (red) and DAPI-stained nuclei (blue) in the ex vivo frozen section of the uterus. (c) Developing a mouse model with endometrial injury and assessment of embryo implantation post-treatment. A schematic diagram shows mouse groupings and detection methods. The endometrial receptivity of five treatment groups is evaluated by counting implanted embryos. *P < 0.05, ** P < 0.01, **** P < 0.0001, n = 8. (d) The HUVECs (human umbilical vein endothelial cells) angiogenesis assay involved co-culturing HP-MSCs with HUVECs, followed by placement of HUVECs in Matrigel-coated u-slides after 24 h for a tube formation assay, with images illustrating tube formation in both control and HP-MSCs groups. Reprinted from Ref [129], open access article distributed under the terms and conditions of the Creative Commons CC BY license
In another study, Kim and colleagues [199] demonstrated the regenerative benefits of HA hydrogel when applied to isolated endometrial stromal cells (EMSCs) in a murine model of uterine infertility [199]. The results indicated that treatment with the hydrogel resulted in a reduction in fibrous tissue and an enhancement in the thickness of the endometrium within two weeks. The regenerated endometrium showed better functionality, leading to successful live births of offspring in the treated area using HA hydrogel [199].
Also, in a study by Zhang et al. [131], the focus was on IUA resulting from endometrial damage [131]. They developed an injectable hydrogel composed of oxidized hyaluronic acid (HA-CHO) and hydrazide-grafted gelatin (Gel-ADH) containing human umbilical cord mesenchymal stem cells (hUCMSCs), demonstrating good biocompatibility. The efficacy of this treatment for endometrial damage was evaluated using a mouse model. The animal model receiving the hydrogel injection with hUCMSC showed significant improvements in endometrial thickness and increased blood vessels and glands in the damaged endometrium compared to the control group. In addition, this treatment significantly decreased the levels of pro-inflammatory factors such as IL-1β and IL-6. It also reduced endometrial fibrosis while increasing the levels of an anti-inflammatory factor (IL-10). These findings suggest that the treatment improved the endometrium’s receptivity to a fetus, leading to successful pregnancy and delivery in mice with endometrial damage. Overall, this research indicates that the injectable hydrogel loaded with hUCMSCs holds excellent promise as a therapeutic approach for the fast recovery of endometrial injuries and presents a valuable biomaterial for regenerative medicine applications [131].
Similarly, Liu and colleagues introduced a novel method aimed at safeguarding the endometrium from damage after intrauterine surgery and addressing the recurrence of AS [125]. They achieved this using a crosslinked hyaluronic acid gel loaded with mesenchymal stem cell secretome (MSC-Sec), which acts as a sustained-release system [125]. Investigations have revealed that MSC-Sec could enhance the proliferation and angiogenesis of the endometrium. In vivo experiments utilizing a rat model of uterine injury displayed that rats treated with MSC-Sec-loaded HA gel exhibited significant improvements in endometrial thickness compared to controls, and the fertility of the testing rats was restored [125]. Moreover, Wenbo et al. [21] reported on chitosan-heparin hydrogels designed for the controlled release of Stromal cell-derived factor-1α (SDF-1α) for intrauterine adhesion treatment [21]. They performed FTIR, SEM, and swelling tests to analyze the composition, morphology, and stability of the hydrogels. Additionally, they evaluated the in vitro release profile using PBS solutions. The study successfully established a mouse model of uterine damage through uterine cort [21].
Zhang et al. [200] also used heparin in a study concentrating on the new strategy for treating IUA. In this regard, they utilized 17β-estradiol heparin-poloxamer thermo-sensitive hydrogel (HP hydrogel) [200]. This poloxamer hydrogel solidifies after cooling and forms a gel state when heated to body temperature. After establishing the IUA model in rats, the efficacy of the heat-sensitive hydrogel (E2-HP) was assessed by injecting it into the injured uterine cavity. According to the findings, E2-HP hydrogel may effectively regenerate the damaged endometrium and inhibit cell apoptosis in the intrauterine adhesion model. As a result, they introduced E2-HP hydrogel as a potentially effective treatment procedure for women with IUAs [200].
It’s worth mentioning that these hydrogels do more than just serve as a support structure to help prevent scarring in the uterus after endometrial damage. They also function as scaffolds that gradually release specific therapeutics, especially growth factors and medications [22, 201]. In this regard, a study [22] investigated a mucoadhesive hydrogel composed of heparin-modified poloxamer (HP) and ε-polylysine (EPL) to improve drug delivery and endometrial tissue repair post-injury. The findings revealed that EPL enhanced the hydrogel’s properties, with the optimal formulation (EPL-HP-90) showing more excellent adhesion and storage modulus than HP alone. EPL also accelerated releasing keratinocyte growth factor (KGF), improving uterine absorption and endometrial repair. The results indicated increased endometrial cell proliferation, enhanced angiogenesis, and reduced apoptosis, suggesting that EPL-HP hydrogels are more effective for endometrial tissue repair than traditional HP hydrogels [22].
The clinical application of polysaccharide-based hydrogels and related materials has been supported by various studies, demonstrating their effectiveness in addressing uterine-related conditions. These materials have shown significant promise in preventing intrauterine adhesions (IUAs), promoting endometrial repair, and enhancing reproductive outcomes. The following table summarizes key findings from some clinical trials validating these claims (Table 3).
Table 3.
Clinical trials validating polysaccharide-based hydrogels in uterine-related conditions
| Study Material | Application | Key Findings | Reference |
|---|---|---|---|
| Alginate Carboxymethylcellulose Hyaluronic Acid (ACH) Gel | Preventing postoperative IUAs | Comparable to CH gel in adhesion prevention; lower adhesion rates in patients without pre-existing IUAs. | [202] |
| Alginate carboxymethylcellulose hyaluronic acid–(ACH) Gel | Preventing IUAs’ post-vacuum aspiration | Reduced IUA incidence (8.06% vs. 19.35% in controls) | [203] |
| Thermosensitive Gels (Sildenafil) + mucoadhesive polymers as sodium alginate and hydroxyethyl cellulose | Treating endometrial thinning | Improved endometrial thickness and uterine blood flow; novel treatment for anovulatory infertility | [204] |
| Chitosan + IUD | Managing IUAs after hysteroscopic adhesiolysis | Improved adhesion scores, endometrial thickness, and clinical pregnancy rates | [205] |
| Autocross-Linked Hyaluronic Acid (HA) Gel | Preventing IUAs after hysteroscopic adhesiolysis | HA gel outperformed IUD in preventing IUAs and improving adhesion-related outcomes. | [206] |
| EmbryoGlue® (High-HA Concentration Medium) | Enhancing live birth rates in FET (frozen embryo transfer) cycles | Higher live birth rates (60.6% vs. 47.5%) and clinical pregnancy rates (69.5% vs. 57.6%); significant association with live birth rates (OR 1.593; CI 1.170–2.168; P = 0.003). | [207] |
Supporting spermatogenesis with polysaccharide-based hydrogels
Male infertility can stem from a variety of causes [9]. Semen and hormone analysis are the main methods of diagnosing male infertility. Serum hormones examined for this purpose include testosterone, follicle-stimulating hormone (FSH), and pituitary-produced gonadotrophins luteinizing hormone (LH). Measuring these hormones will help determine the cause of infertility. Reduced production of sperm (spermatogenesis) results in an increase in FSH due to the hypothalamic-pituitary-gonadal feedback loop. That’s why hypergonadotropic oligo- or azoospermia is observed in about 60% of infertile men [208].
Spermatogenic failure, the most extreme form of male infertility, usually results in the absence of sperm in the semen (azoospermia). Some men with this disease can produce sperm in certain parts of their testicles. These locations can be extracted for in vitro fertilization to produce healthy offspring. The treatment options for spermatogenic failure-related infertility are numerous and include hormonal, microsurgical, and genetic studies. So, testicular tissue culture in laboratory conditions is necessary to study spermatogenesis and treat male infertility. In addition, innovative research in stem cells seeks to create artificial gametes as a new solution for infertility treatment [209].
Nowadays, male infertility can be treated using assisted reproductive techniques (ART) [9]. Researchers can treat certain forms of spermatogenic failure by growing testicular tissue in vitro, and one tool to combat male infertility involves using spermatogonial stem cells [2]. In recent years, laboratory culture methods for testicular tissue have experienced great changes. These changes include improving the conditions of the cultivation environment regarding temperature, gas content, etc [2]. For young boys undergoing gonadotoxic therapies like radiation therapy or chemotherapy, immature testicular tissue (ITT) transplantation is a potentially effective technique to restore fertility. Research indicates that the quantity of spermatogonia in avascular xenografts of cryopreserved human ITT is notably diminished. For instance, Hydrogels containing VEGF nanoparticles are more effective in improving tissue survival and spermatogonial regeneration. With this approach, in a study performed by Poels et al. [43], the stable release of VEGF from biodegradable and biocompatible hydrogels to preserve testicular tissue in the first days after transplantation to reduce ischemic stress was investigated [43]. In this regard, ITT was embedded in hydrogels containing VEGF nanoparticles to enhance spermatogonial survival, revascularization, and tissue engraftment, and the possibility of an alginate hydrogel to improve cryopreserved tissue engraftment was investigated [43]. This investigation aimed to explore the potential of engineered materials that enhance angiogenesis in avascular testicular tissue grafts by combining growth factor delivery and extracellular matrices. The study involved transplants in five groups: non-encapsulated grafted tissue, fibrin-encapsulated graft, alginate-encapsulated graft, fibrin-VEGF-NP encapsulated graft, and alginate-VEGF-NP encapsulated graft. The integrity of the spermatogenic tube and the re-establishment of blood vessels were evaluated after 5 and 21 days. They found that alginate-encapsulated tissue provides a controlled release of VEGF over a longer duration than fibrin-encapsulated tissue, which results in prolonged angiogenesis simulation. Additionally, alginate’s antioxidant properties aid in the survival of spermatogonia by decreasing reactive oxygen species. This study demonstrated how alginate hydrogel containing nano-encapsulated growth factors could increase the effectiveness of frozen tissue grafting [43].
In another study [2], the gas-liquid interphase technique based on agarose was used to cultivate the testicular tissues of adult mice, methacryloyl gelatin (GelMA), methacryloyl alginate (AlgMA), methacryloyl dextran (DexMA), and each with agarose [2]. Tan et al. [2] then measured the properties of the hydrogels to look into how their physical and biochemical characteristics impact the testicular tissue culture results. The results showed that on day 32, compared to other hydrogels, AlgMA hydrogel had the highest density of spermatocyte cells and high integrity of seminiferous tubes. The large volume of water in the AlgMA hydrogel probably accounts for these results, as it reduces the harmful effects of oxygen on the testicular tissue. Also, the results of the culture of testicular tissues on DexMA hydrogel showed that more testosterone is produced than in other groups [2].
In conclusion, recent research shows that using hydrogels containing growth factors and improving testicular tissue culture methods can help the regeneration and survival of spermatogenic cells and bring new hope for restoring fertility in men.
Drug/cell delivery systems using hydrogels for infertility treatment
There are many different drug delivery systems and devices being used and developed for diagnosing and treating infertility. These forms of medication vary greatly, including traditional solid forms such as tablets, capsules, and suppositories, semi-solid forms like hydrogels and creams, and liquid forms such as solutions and injections. More advanced delivery methods are also being explored, including micro-particles, nanoparticles, and systems for delivering stem cells [188]. Some of the significant challenges in drug delivery involve confirming that the medication reaches the right target, minimizing side effects throughout the body, attaining a sustained release of the drug, and preserving its effectiveness. It has been mentioned that biomaterials can replicate the natural tissue of the endometrium and, when designed properly, can release drugs, growth factors, and bioactive chemicals under controlled conditions to promote tissue regeneration and improve therapeutic outcomes. As a result, they can lead to more accurate drug delivery and improved treatment results [200, 210]. Local drug delivery systems have significant potential in this area. Several studies have explored polysaccharide-based hydrogel drug delivery systems aimed at treating infertility [156].
Keratinocyte growth factor (KGF) is vital in repairing epithelial tissues. Xu et al. [20] used an HP hydrogel combined with KGF (KGF-HP) as a matrix for the delivery of KGF and the treatment of IUA [20] (Fig. 8). The study examined the rheology of KGF HP hydrogel, highlighting its temperature sensitivity. The hydrogel remains in a liquid state at 4 °C, which allows for easy application to the wound. Upon the temperature rising to body temperature (37 °C), the hydrogel transforms into a solid, effectively covering the wound surface. This transformation helps minimize the risk of bacterial infection and conserves medication effectively. In vitro release demonstrated the stable release of KGF from HP hydrogels. The application of KGF-HP hydrogel led to a significant improvement in the morphology and functionality of the damaged uterus [20].
Fig. 8.
(a) Temperature-dependent appearance of KGF-HP hydrogels at a KGF concentration of 2.5 mg/ml. (b) Fluorescence imaging of the rat’s intact uterus displaying a strong fluorescence signal in the injured area (the right horn of the uterus) after administering the KGF-HP hydrogel. (c) Representative 2D ultrasound images of rat uteri from different groups: (i) the sham group, (ii) the vehicle-treated group (IUA group), (iii) the group treated with KGF solution, and (iv) the group treated with KGF-HP hydrogel, indicating a recovery in the echogenic signal within the endometrial tissue and a prominent white line in the endometrium seven days after treatment with either KGF solution or KGF-HP hydrogel, signifying effective recovery of the damaged endometrial structure. (d) Representative images of embryo implantation in rats using various formulations, the injured zone indicated by the red arrow. (e) Pregnancy outcomes at 90 days after surgery in the sham-operated, IUA, KGF, and KGF-HP treatment groups (*p < 0.05; n = 6). Reprinted from Ref [20], open access article distributed under the terms and conditions of the Creative Commons CC BY license
Also, Cai et al. [196] conducted a study aimed at the fabrication of a new biodegradable porous scaffold with the ability to load drugs based on a microfluidic droplet template, which was a combination of gelatin methacrylate (GelMA) and sodium alginate as two biocompatible materials. These scaffolds, which are porous and interconnected, created an ideal space for drug delivery and release. This feature enables the system to support endometrial repair effectively. The design of this scaffold allows it to adapt to the shape of the uterine cavity, and due to the flexibility, mechanical properties, and porous structure of this scaffold, it can be compressed and transferred to the uterus through the vagina. Experiments were performed using a rat model of IUAs to evaluate the efficiency of this scaffold. Based on these findings, these scaffolds can promote neovascularization, help regenerate injured tissue, and repair the endometrium. These findings indicate that drug-containing scaffolds may be a suitable option for treating of intrauterine adhesions after surgery [196].
Qi et al. [21] conducted another investigation in 2020, wherein they used a mild process to prepare a chitosan-heparin hydrogel loaded with SDF-1α, a chemokine protein that can recruit endogenous cells to treat intrauterine adhesions in the rat model. In this study, hydrogels were employed for the controlled release of SDF-1α in vivo. The results of immunofluorescence and immunohistochemistry staining indicated that during the treatment process, hematopoietic stem cells were recruited to the damaged area, leading to improved wound recovery. The outcomes of this study showed that using chitosan-heparin hydrogel as a carrier for the local delivery of SDF-1α can effectively help in the precise repair of the damaged uterus in rats. This drug delivery method can be recommended as an appropriate option for treating uterine adhesions [21].
In 2022, Xin et al. [197] conducted another study aimed at treating IUA as a cell-free therapeutic strategy, where they utilized HA hydrogel to deliver apoptotic bodies (ApoBDs) derived from mesenchymal stem cells [197]. ApoBDs are a specific type of extracellular vesicle released during apoptosis. They include vital biomolecules that maintain homeostasis, support tissue regeneration, and influence angiogenesis, cell proliferation, and macrophage immunomodulation [5, 211, 212]. In this study, combining ApoBDs with HA hydrogels as a delivery system resulted in enhanced sustained release and retention of ApoBDs. To examine the therapeutic effects of this construct on endometrial regeneration, rat IUA models and mouse models of acute endometrial injury were utilized. The results showed a notable increase in endometrial thickness following a local injection of ApoBD-containing HA hydrogel. This enhancement in the endometrial structure contributed to the restoration of fertility [197].
Therefore, effective drug delivery plays a crucial role in addressing infertility issues. Recently, stem cells have also gained recognition for their potential to diagnose and treat these conditions accurately. In diseases related to infertility, stem cells have also been widely used, particularly in today’s world, where numerous couples are facing infertility issues [213]. For treating male and female genital abnormalities, one of the fertility recovery methods is the treatment based on stem cells, including embryonic stem cells, bone marrow mesenchymal stem cells, and mesenchymal stem cells derived from the umbilical cord. This process focuses on introducing cellular materials into patients’ bodies using methods like injection, transplantation, or implantation [213]. Integrating cells into a hydrogel matrix has recently gained popularity for therapeutic applications, particularly in infertility treatment. In a study, Ghasemi et al. [214] optimized the retinoic acid (RA-a vitamin A metabolite) dose to differentiate human endometrial mesenchymal stem cells (hEnMSCs) into oocyte-like cells [214]. They evaluated the expression level of markers associated with oocyte-like cell growth before and after embedding in alginate hydrogel, comparing 2D and 3D cell culture systems. Biocompatibility of alginate hydrogel produced on differentiated cells was confirmed. Also, the results showed that hEnMSCs could differentiate into oocyte-like cells after five days of encapsulation in alginate hydrogel with an adjusted dose of RA [214].
Overall, using polysaccharide-based hydrogels as innovative delivery systems can significantly enhance treating infertility and aid in regenerating damaged tissues. These systems allow for the controlled release of drugs and growth factors, leading to better patient outcomes.
On the other hand, studies underscore the complexity of infertility, influenced by various factors. While polysaccharide-based hydrogels show promise for regenerating endometrial tissue and facilitating cell interactions, significant improvements in fertility outcomes rely on many other elements, such as hormonal balance, immune responses, and various systemic factors. For instance, it has been found that alginate-fibrin hydrogels, when enriched with Melatonin and endothelial cells, boosted blood vessel formation and reduced scarring in transplanted ovarian tissues, helping with cell survival and overall tissue function [94].
However, to achieve consistent positive results in clinical settings, it is necessary to refine the properties of these hydrogels further and gain more insights into how they interact with surrounding tissues. Future research should aim to combine these hydrogels with systemic treatments to address the root causes of infertility.
Challenges and limitations of polysaccharide-based hydrogels in infertility treatment and future directions
The findings are encouraging, but they also highlight the complexities involved in using polysaccharide-based hydrogels for treating infertility, which need more discussion. Although notable progress has been made in regenerating endometrial tissue and improving embryo implantation rates, achieving reliable enhancements in overall fertility outcomes remains difficult. Such challenges stem from the fact that many different factors influence infertility, while polysaccharide-based hydrogels address some but not all contributing factors.
One notable gap in current research is the insufficient clinical studies on the use of polysaccharide-based hydrogels in treating infertility. The majority of the existing studies are preclinical, and further clinical trials are necessary to evaluate the safety and efficacy of these hydrogels in real-world applications. The lack of data makes it essential to approach the translation of these findings into clinical practice with caution.
So, comprehensive preclinical and clinical investigations are necessary to evaluate the feasibility and efficacy of these innovative therapies. Moreover, while some recent studies have begun to explore various aspects of these hydrogels, further research is needed to refine their application. Also, they face other significant challenges, such as rapid degradation, mechanical limitations, immunogenicity, and diverse environmental conditions [215, 216]. Many natural polysaccharide hydrogels, such as those derived from hyaluronic acid or pectin, do not exhibit sufficient mechanical strength. Therefore, they must be improved by physical crosslinking or structural modification for long-term or load-bearing applications [117, 216]. However, the high biodegradability of natural hydrogels often leads to rapid degradation in vivo, which can compromise their therapeutic efficacy. Hybridization with synthetic materials and chemical crosslinking can overcome this problem but may raise biocompatibility concerns. Notably, the performance of these hydrogels can vary significantly depending on the physiological environment, such as temperature, pH, or enzymatic activity. In addition, modified polysaccharides or those obtained from specific sources may induce immune responses, limiting their usefulness in clinical settings. These hydrogels are effective for specific purposes, such as tissue regeneration or drug delivery, but they may not adequately address the complexity of infertility. In the end, scaling up the production of these hydrogels while ensuring consistent quality and performance for large-scale clinical use is an ongoing challenge.
Therefore, suitable strategies to develop and apply polysaccharide-based hydrogels should become standard protocols. Future research should focus on creating hybrid hydrogels. These hydrogels would combine synthetic and natural components to achieve a balance between mechanical strength, stability, and biocompatibility. Advanced fabrication techniques, such as 3D bio-printing using polysaccharide bio-inks to create tissue constructs and personalized organoids, could improve the precision and functionality of these materials in reproductive medicine and transform infertility treatment. In vivo testing in various physiological environments helps assess their performance and stability. In this regard, researchers are developing targeted drug delivery systems for the controlled release of growth factors and hormones. Addressing these challenges, future studies can optimize polysaccharide-based hydrogels, maximizing their potential in regenerative medicine and infertility treatment.
Conclusion
In conclusion, infertility is a complicated health issue that requires novel solutions, particularly in the realm of female reproductive health. Current standard treatments are limited in effectively repairing damaged tissues and enhancing reproductive capabilities, and new therapeutic options are necessary. Tissue engineering and regenerative medicine hold great promise for addressing infertility and various reproductive health issues. Utilizing innovative biomaterials, particularly polysaccharide-based hydrogels, offers an exciting possibility to develop effective treatments for endometrial injuries and other infertility-related problems. In this context, polysaccharide-based hydrogels present a bioactive micro/nano environment for tissue repair and regeneration. These properties allow for the controlled release of therapeutic agents with natural tissue characteristics. Integrating hydrogels into reproductive medicine will significantly improve clinical outcomes as research progresses.
In this regard, this review brings together findings from different studies on polysaccharide-based hydrogels, developing a clearer understanding of their properties and uses in addressing various infertility-related issues. Organizing these hydrogels according to their biochemical and mechanical traits helps researchers choose the most appropriate materials for specific therapeutic needs. On the other hand, researchers should interpret the current findings cautiously due to the limitations discussed. The variability in hydrogel performance under physiological conditions, the lack of large-scale clinical data, and the multifactorial nature of infertility present essential challenges that need to be addressed.
Overall, the insights from this review highlight gaps in current clinical applications and propose new research opportunities. These include developing hybrid hydrogels for personalized therapies, enhancing biocompatibility, and conducting more rigorous clinical studies. Further research and development in this field could result in breakthroughs in infertility treatments, providing new hope for countless couples dealing with reproductive issues.
Acknowledgements
We would like to thank the Department of Tissue Engineering, Faculty of Advanced Medical Science of Tabriz University for all the support provided.
The authors acknowledge utilizing the AI-based tool Grammarly exclusively for language editing, clarity enhancement, and grammatical corrections in preparing this manuscript. The intellectual content, conceptualization, and writing were entirely performed by the authors. Grammarly’s assistance was limited to refining the language and ensuring the manuscript’s overall readability.
Abbreviations
- IUA
Intrauterine adhesions
- AS
Asherman’s syndrome
- ART
Assisted reproductive techniques
- TERM
Tissue Engineering and Regenerative Medicine
- iPSCs
Induced pluripotent stem cells
- WHO
World Health Organization
- REPROTEN
Reproductive tissue engineering
- ED
Erectile dysfunction
- SCT
Stem cell therapy
- iPS
Induced stem cells
- ES
Embryonic stem cells
- ECM
Extracellular matrix
- HA
Hyaluronic acid
- Alg
Alginate
- Fib
Fibrin
- Mel
Melatonin
- ECs
Endothelial cells
- PEGDA
Poly (ethylene glycol) diacrylate
- SA
Sodium alginate
- GAG
Glycosaminoglycan
- HP
MSCs-Human placenta-derived mesenchymal stem cells
- HA-CHO
Hydrogel composed of oxidized hyaluronic acid
- Gel-ADH
Hydrazide-grafted gelatin
- hUCMSCs
Human umbilical cord mesenchymal stem cells
- VEGF
Vascular endothelial growth factor
- bFGF
Fibroblast growth factor
- HABH
Hyaluronic acid hydrogel scaffold
- SDF-1α
Stromal cell-derived factor-1α
- HASH
Thiolate hyaluronic acid
- CSH
Thiolate chitosan
- Alkyl-β-CD
Alkylatedβ-cyclodextrins
- CSHS
Chitosan-thiolate hyaluronic hydrogel
- PDA
Polydopamine
- CMCS
Carboxymethyl chitosan
- Arg
Arginine
- E2-HP
Heat-sensitive hydrogel
- HP
Heparin-modified poloxamer
- EPL
ε-polylysine
- KGF
Keratinocyte growth factor
- POF
Premature ovarian failure
- OvaECM
Ovarian cortex extracellular matrix
- POI
Premature ovarian insufficiency
- hGCs
Human granulosa cells
- AO
Artificial ovary
- CTP
Chitin-based
- hAECs
Human amniotic epithelial cells
- CM
Conditioned medium
- SA-BG
Sodium alginate-bioglass
- EC
Endometrial bilayer
- Alg-HA
Alginate-hyaluronic acid hydrogel
- EBB
Extrusion-based bioprinting
- EECs
Endometrial epithelial cells
- ESCs
Endometrial stromal cells
- hiMSCs
Human induced pluripotent stem cells
- MSC-Sec
Mesenchymal stem cell secretome
- EMSCs
Endometrial stromal cells
- FSH
Follicle-stimulating hormone
- LH
Luteinizing hormone
- ART
Assisted reproductive techniques
- ITT
Immature testicular tissue
- GelMA
Methacryloyl gelatin
- AlgMA
Methacryloyl alginate
- DexMA
Methacryloyl dextran
- KGF
Keratinocyte growth factor
- ApoBDs
Apoptotic bodies derived from mesenchymal stem cells
- RA
Retinoic acid
Author contributions
Conceptualization, A.R.D.B. (Azizeh Rahmani Del Bakhshayesh); writing—original draft preparation, M.G.N., S.B., S.B., M.R.D.B. and A.R.D.B. (Azizeh Rahmani Del Bakhshayesh); writing—review and editing, M.G.N., S.B., A.C.P.S., M.R.D.B., N.A.G., S.F.K., D.G. and A.R.D.B. (Azizeh Rahmani Del Bakhshayesh); supervision, A.R.D.B. (Azizeh Rahmani Del Bakhshayesh); project administration, A.R.D.B. (Azizeh Rahmani Del Bakhshayesh). All authors have read and approved the final version of the manuscript.
Funding
The author(s) received no financial support.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jahanbani Y, et al. Scaffold-based tissue engineering approaches in treating infertility. Life Sci. 2020;240:117066. [DOI] [PubMed] [Google Scholar]
- 2.Tan J, et al. Application of photocrosslinked gelatin, alginate and dextran hydrogels in the in vitro culture of testicular tissue. Int J Biol Macromol. 2024;260:129498. [DOI] [PubMed] [Google Scholar]
- 3.Critchley HOD, et al. Menstruation: science and society. Am J Obstet Gynecol. 2020;223(5):624–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fitzgerald HC, Schust DJ. E.J.B.o.r. Spencer. Vitro Models Hum Endometrium: Evol Application Women’s Health. 2021;104(2):282–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai G et al. Recent developments in biomaterial-based hydrogel as the delivery system for repairing endometrial injury. 2022. 10: p. 894252. [DOI] [PMC free article] [PubMed]
- 6.Jahanbani Y, et al. Scaffold-based Tissue Eng Approaches Treat Infertility. 2020;240:117066. [DOI] [PubMed] [Google Scholar]
- 7.Johary J et al. Efficacy of Estrogen therapy in patients with intrauterine adhesions: systematic review. 2014. 21(1): pp. 44–54. [DOI] [PubMed]
- 8.Lin X-N et al. Randomized, controlled trial comparing the efficacy of intrauterine balloon and intrauterine contraceptive device in the prevention of adhesion reformation after hysteroscopic adhesiolysis. 2015. 104(1): pp. 235–40. [DOI] [PubMed]
- 9.Ghanbari E, et al. Novel therapeutic approaches of tissue engineering in male infertility. Cell Tissue Res. 2020;380(1):31–42. [DOI] [PubMed] [Google Scholar]
- 10.Zhao W et al. Delivery of stromal cell-derived factor 1α for in situ tissue regeneration. 2017. 11: pp. 1–12. [DOI] [PMC free article] [PubMed]
- 11.Berradi A, et al. A comprehensive review of Polysaccharide-Based hydrogels as promising biomaterials. Polymers. 2023;15(13):2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farasati Far B et al. A review on biomedical application of Polysaccharide-Based hydrogels with a focus on drug delivery systems. Polym (Basel), 2022. 14(24). [DOI] [PMC free article] [PubMed]
- 13.Taghipour YD, et al. The application of hydrogels based on natural polymers for tissue engineering. Curr Med Chem. 2020;27(16):2658–80. [DOI] [PubMed] [Google Scholar]
- 14.Sanjanwala D, et al. Polysaccharide-based hydrogels for medical devices, implants and tissue engineering: A review. Int J Biol Macromol. 2024;256:128488. [DOI] [PubMed] [Google Scholar]
- 15.Lopez-Sanchez P, et al. Impact of glucose on the nanostructure and mechanical properties of calcium-alginate hydrogels. Gels. 2022;8(2):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang X, et al. Emerging biomaterials for reproductive medicine. Eng Regeneration. 2021;2:230–45. [Google Scholar]
- 17.Miles JR, et al. In vitro Porcine blastocyst development in three-dimensional alginate hydrogels. Mol Reprod Dev. 2017;84(9):775–87. [DOI] [PubMed] [Google Scholar]
- 18.Lv H, et al. Hydrogel, a novel therapeutic and delivery strategy, in the treatment of intrauterine adhesions. J Mater Chem B. 2021;9(33):6536–52. [DOI] [PubMed] [Google Scholar]
- 19.Arjmand F, et al. Extended culture of encapsulated human blastocysts in alginate hydrogel containing decidualized endometrial stromal cells in the presence of melatonin. Mol Biotechnol. 2016;58:684–94. [DOI] [PubMed] [Google Scholar]
- 20.Xu H-L, et al. Temperature-sensitive heparin-modified poloxamer hydrogel with affinity to KGF facilitate the morphologic and functional recovery of the injured rat uterus. Drug Delivery. 2017;24(1):867–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wenbo Q, et al. Controlled releasing of SDF-1α in chitosan-heparin hydrogel for endometrium injury healing in rat model. Int J Biol Macromol. 2020;143:163–72. [DOI] [PubMed] [Google Scholar]
- 22.Xu H-L, et al. Dual regulations of thermosensitive heparin–poloxamer hydrogel using ε-polylysine: bioadhesivity and controlled KGF release for enhancing wound healing of endometrial injury. ACS Appl Mater Interfaces. 2017;9(35):29580–94. [DOI] [PubMed] [Google Scholar]
- 23.Montoya-Botero P, Polyzos NP. The endometrium during and after ovarian hyperstimulation and the role of segmentation of infertility treatment. Volume 33. Best Practice & Research Clinical Endocrinology & Metabolism; 2019. pp. 61–75. 1. [DOI] [PubMed]
- 24.Agarwal A, et al. A unique view on male infertility around the Globe. Reproductive Biology Endocrinol. 2015;13:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sethi P, et al. Prevalence of psychiatric morbidity in females amongst infertile couples-A hospital based report. J Clin Diagn Research: JCDR. 2016;10(7):VC04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moridi A, et al. Etiology and risk factors associated with infertility. Int J Women’s Health Reprod Sci. 2019;7(3):346–53. [Google Scholar]
- 27.Obeagu EI, Njar VE, Obeagu GU. Infertility: prevalence and consequences. Int J Curr Res Chem Pharm Sci. 2023;10(7):43–50. [Google Scholar]
- 28.Bhattacharya I, Sharma SS, Majumdar SS. Etiology of male infertility: an update. Reproductive Sci. 2024;31(4):942–65. [DOI] [PubMed] [Google Scholar]
- 29.Ferlin A, et al. Male infertility: role of genetic background. Reprod Biomed Online. 2007;14(6):734–45. [DOI] [PubMed] [Google Scholar]
- 30.O’brien KLf., Varghese AC, Agarwal A, editors. The genetic causes of male factor infertility: a review. Fertility and sterility, 2010. 93(1): pp. 1–12. [DOI] [PubMed]
- 31.Luk BH-K, Loke AY. The impact of infertility on the psychological well-being, marital relationships, sexual relationships, and quality of life of couples: A systematic review. J Sex Marital Ther. 2015;41(6):610–25. [DOI] [PubMed] [Google Scholar]
- 32.Peng G, Liu H, Fan Y. Biomaterial scaffolds for reproductive tissue engineering. Ann Biomed Eng. 2017;45:1592–607. [DOI] [PubMed] [Google Scholar]
- 33.Strauss JF, et al. Yen & Jaffe’s Reproductive Endocrinology-E-Book: Physiology, Pathophysiology, and Clinical Management. Elsevier Health Sciences; 2023.
- 34.Gargus ES, et al. Engineered reproductive tissues. Nat Biomedical Eng. 2020;4(4):381–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leonel EC, et al. New solutions for old problems: how reproductive tissue engineering has been revolutionizing reproductive medicine. Ann Biomed Eng. 2023;51(10):2143–71. [DOI] [PubMed] [Google Scholar]
- 36.Favre-Inhofer A, et al. Uterine transplantation: review in human research. J Gynecol Obstet Hum Reprod. 2018;47(6):213–21. [DOI] [PubMed] [Google Scholar]
- 37.Rodriguez KM, Pastuszak AW. A history of penile implants. Translational Androl Urol. 2017;6(Suppl 5):S851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Girsdansky J, Newman HF. Use of a vitallium testicular implant. Am J Surg. 1941;53(3):514. [Google Scholar]
- 39.Zeilmaker GH, et al. Two pregnancies following transfer of intact frozen-thawed embryos. Fertil Steril. 1984;42(2):293–6. [DOI] [PubMed] [Google Scholar]
- 40.Palermo G, et al. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet. 1992;340(8810):17–8. [DOI] [PubMed] [Google Scholar]
- 41.Raya-Rivera AM, et al. Tissue-engineered autologous vaginal organs in patients: a pilot cohort study. Lancet. 2014;384(9940):329–36. [DOI] [PubMed] [Google Scholar]
- 42.Laronda MM, et al. A bioprosthetic ovary created using 3D printed microporous scaffolds restores ovarian function in sterilized mice. Nat Commun. 2017;8(1):15261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poels J, et al. Transplantation of testicular tissue in alginate hydrogel loaded with VEGF nanoparticles improves spermatogonial recovery. J Controlled Release. 2016;234:79–89. [DOI] [PubMed] [Google Scholar]
- 44.Amorim CA, et al. Survival of human pre-antral follicles after cryopreservation of ovarian tissue, follicular isolation and in vitro culture in a calcium alginate matrix. Hum Reprod. 2009;24(1):92–9. [DOI] [PubMed] [Google Scholar]
- 45.Vanacker J, et al. Transplantation of an alginate–matrigel matrix containing isolated ovarian cells: first step in developing a biodegradable scaffold to transplant isolated preantral follicles and ovarian cells. Biomaterials. 2012;33(26):6079–85. [DOI] [PubMed] [Google Scholar]
- 46.Laronda MM, et al. Initiation of puberty in mice following decellularized ovary transplant. Biomaterials. 2015;50:20–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiao S, et al. A microfluidic culture model of the human reproductive tract and 28-day menstrual cycle. Nat Commun. 2017;8(1):14584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gholami K, et al. Organ culture of seminiferous tubules using a modified soft agar culture system. Stem Cell Res Ther. 2018;9:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nishiguchi A, et al. In vitro placenta barrier model using primary human trophoblasts, underlying connective tissue and vascular endothelium. Biomaterials. 2019;192:140–8. [DOI] [PubMed] [Google Scholar]
- 50.Kessler M, et al. The Notch and Wnt pathways regulate stemness and differentiation in human fallopian tube organoids. Nat Commun. 2015;6(1):8989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu X, et al. Bioadaptability of biomaterials: aiming at precision medicine. Matter. 2021;4(8):2648–50. [Google Scholar]
- 52.Li J, et al. Human chorionic plate-derived mesenchymal stem cells transplantation restores ovarian function in a chemotherapy-induced mouse model of premature ovarian failure. Stem Cell Res Ther. 2018;9:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou R, et al. The roles and mechanisms of Leydig cells and myoid cells in regulating spermatogenesis. Cell Mol Life Sci. 2019;76:2681–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kapoor D, Leech J, Yap W. Use of tunica vaginalis patch graft for repair of traumatic testicular rupture. Urology. 1992;40(4):374–5. [DOI] [PubMed] [Google Scholar]
- 55.Molokwu CN, Doull RI, Townell NH. A novel technique for repair of testicular rupture after blunt trauma. Urology. 2010;76(4):1002–3. [DOI] [PubMed] [Google Scholar]
- 56.Liu F, et al. Cell and biomaterial-based approaches to uterus regeneration. Regenerative Biomaterials. 2019;6(3):141–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Vocht DE, et al. A systematic review on cell-seeded tissue engineering of penile corpora. J Tissue Eng Regen Med. 2018;12(3):687–94. [DOI] [PubMed] [Google Scholar]
- 58.Adjei E, Saeinasab M, Sefat F. Tissue engineering strategies to treat female infertility, in Regenerative medicine in the genitourinary system. Elsevier; 2024. pp. 299–324.
- 59.Sittadjody S, et al. Engineered multilayer ovarian tissue that secretes sex steroids and peptide hormones in response to gonadotropins. Biomaterials. 2013;34(10):2412–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ouni E, et al. A draft map of the human ovarian proteome for tissue engineering and clinical applications. Mol Cell Proteom. 2019;18:S159–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gosden R. Restitution of fertility in sterilized mice by transferring primordial ovarian follicles. Hum Reprod. 1990;5(2):117–22. [DOI] [PubMed] [Google Scholar]
- 62.Tamadon A, et al. Efficient biomaterials for tissue engineering of female reproductive organs. Tissue Eng Regenerative Med. 2016;13:447–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pors S, et al. Towards an artificial ovary: grafting preantral follicles on decellularized human ovarian tissue. Hum Reprod. 2018;33:i39. [Google Scholar]
- 64.Ferenczy A. Anatomy and histology of the uterine corpus. Blaustein’s pathology of the female genital tract. Springer; 1994. pp. 327–66.
- 65.Sadri-Ardekani H, Atala A. Regenerative medicine for the treatment of reproductive system disorders: current and potential options. Adv Drug Deliv Rev. 2015;82:145–52. [DOI] [PubMed] [Google Scholar]
- 66.Turco MY, et al. Long-term, hormone-responsive organoid cultures of human endometrium in a chemically defined medium. Nat Cell Biol. 2017;19(5):568–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tiemann T, et al. Towards uterus tissue engineering: a comparative study of sheep uterus decellularisation. Mol Hum Reprod. 2020;26(3):167–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yao Q et al. Exploiting crosslinked decellularized matrix to achieve uterus regeneration and construction. Artificial Cells, Nanomedicine, and Biotechnology, 2020. 48(1): pp. 218–229. [DOI] [PubMed]
- 69.Yucer N, et al. Directed differentiation of human induced pluripotent stem cells into fallopian tube epithelium. Sci Rep. 2017;7(1):10741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Parasar P, et al. Differentiating mouse embryonic stem cells express markers of human endometrium. Reproductive Biology Endocrinol. 2017;15:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Francés-Herrero E, et al. Development of decellularized oviductal hydrogels as a support for rabbit embryo culture. Reproductive Sci. 2021;28:1644–58. [DOI] [PubMed] [Google Scholar]
- 72.De Filippo RE, Yoo JJ, Atala A. Engineering of vaginal tissue in vivo. Tissue Eng. 2003;9(2):301–6. [DOI] [PubMed] [Google Scholar]
- 73.Zhu L, et al. Anatomic and sexual outcomes after vaginoplasty using tissue-engineered biomaterial graft in patients with Mayer‐Rokitansky‐Küster‐Hauser syndrome: A new minimally invasive and effective surgery. J Sex Med. 2013;10(6):1652–8. [DOI] [PubMed] [Google Scholar]
- 74.Panici PB, et al. Autologous in vitro cultured vaginal tissue for vaginoplasty in women with Mayer-Rokitansky-Küster-Hauser syndrome: anatomic and functional results. J Minim Invasive Gynecol. 2015;22(2):205–11. [DOI] [PubMed] [Google Scholar]
- 75.Durairajanayagam D et al. Sperm biology from production to ejaculation. Unexplained Infertility: Pathophysiology, Evaluation and Treatment, 2015: pp. 29–42.
- 76.Kargar-Abarghouei E, et al. Characterization, recellularization, and transplantation of rat decellularized testis scaffold with bone marrow-derived mesenchymal stem cells. Stem Cell Res Ther. 2018;9:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee JH, et al. In vitro spermatogenesis by three-dimensional culture of rat testicular cells in collagen gel matrix. Biomaterials. 2006;27(14):2845–53. [DOI] [PubMed] [Google Scholar]
- 78.Gassei K, Ehmcke J, Schlatt S. Initiation of testicular tubulogenesis is controlled by neurotrophic tyrosine receptor kinases in a three-dimensional Sertoli cell aggregation assay. Reproduction. 2008;136(4):459. [DOI] [PubMed] [Google Scholar]
- 79.Meng L, et al. Tissue-engineered tubular substitutions for urinary diversion in a rabbit model. Experimental Biology Med. 2016;241(2):147–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Campbell GR, et al. The peritoneal cavity as a bioreactor for tissue engineering visceral organs: bladder, uterus and Vas deferens. J Tissue Eng Regen Med. 2008;2(1):50–60. [DOI] [PubMed] [Google Scholar]
- 81.de Souza DAS, et al. Congenital bilateral absence of the Vas deferens as an atypical form of cystic fibrosis: reproductive implications and genetic counseling. Andrology. 2018;6(1):127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bretan PN Jr. History of the prosthetic treatment of impotence. Urol Clin North Am. 1989;16(1):1–5. [PubMed] [Google Scholar]
- 83.Kessler A, et al. The global prevalence of erectile dysfunction: a review. BJU Int. 2019;124(4):587–99. [DOI] [PubMed] [Google Scholar]
- 84.Chen K-L et al. Bioengineered corporal tissue for structural and functional restoration of the penis. Proceedings of the National Academy of Sciences, 2010. 107(8): pp. 3346–3350. [DOI] [PMC free article] [PubMed]
- 85.Kershen RT, et al. Reconstitution of human corpus cavernosum smooth muscle in vitro and in vivo. Tissue Eng. 2002;8(3):515–24. [DOI] [PubMed] [Google Scholar]
- 86.Falke G, et al. Formation of corporal tissue architecture in vivo using human cavernosal muscle and endothelial cells seeded on collagen matrices. Tissue Eng. 2003;9(5):871–9. [DOI] [PubMed] [Google Scholar]
- 87.Wang B, et al. Recent advances in stem cell therapy for erectile dysfunction: a narrative review. Expert Opin Biol Ther. 2023;23(6):565–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang J, et al. Application of bioactive hydrogels for functional treatment of intrauterine adhesion. Front Bioeng Biotechnol. 2021;9:760943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Varaprasad K, et al. A mini review on hydrogels classification and recent developments in miscellaneous applications. Mater Sci Engineering: C. 2017;79:958–71. [DOI] [PubMed] [Google Scholar]
- 90.Chamkouri H, Chamkouri M. A review of hydrogels, their properties and applications in medicine. Am J Biomed Sci Res. 2021;11(6):485–93. [Google Scholar]
- 91.Gosecka M, Gosecki M. Antimicrobial polymer-based hydrogels for the intravaginal therapies—engineering considerations. Pharmaceutics. 2021;13(9):1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jacob S, et al. Emerging role of hydrogels in drug delivery systems, tissue engineering and wound management. Pharmaceutics. 2021;13(3):357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Frances-Herrero E, et al. Bioengineering trends in female reproduction: a systematic review. Hum Reprod Update. 2022;28(6):798–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Izadpanah M, et al. Melatonin and endothelial cell-loaded alginate-fibrin hydrogel promoted angiogenesis in rat cryopreserved/thawed ovaries transplanted to the heterotopic sites. J Biol Eng. 2023;17(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zarnaghi MR, et al. Assessment of apoptosis and oxidative stress in cryopreserved ovary after grafting in fibrin-alginate scaffold with endothelial cells and melatonin in Wistar rats. J Gynecol Obstet Hum Reprod. 2024;53(9):102828. [DOI] [PubMed] [Google Scholar]
- 96.Xiang D, et al. Artificial ovaries constructed from biodegradable chitin-based hydrogels with the ability to restore ovarian endocrine function and alleviate osteoporosis in ovariectomized mice. Reproductive Biology Endocrinol. 2023;21(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Matsushige C, et al. RGD-modified dextran hydrogel promotes follicle growth in three-dimensional ovarian tissue culture in mice. Theriogenology. 2022;183:120–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Coello A, et al. A combination of hydroxypropyl cellulose and Trehalose as supplementation for vitrification of human oocytes: a retrospective cohort study. J Assist Reprod Genet. 2016;33:413–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sharma R, et al. A critical review on classified excipient Sodium-Alginate-Based hydrogels: modification, characterization, and application in soft tissue engineering. Gels. 2023;9(5):430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sarker B, et al. Alginate-based hydrogels with improved adhesive properties for cell encapsulation. Int J Biol Macromol. 2015;78:72–8. [DOI] [PubMed] [Google Scholar]
- 101.Highley CB, Prestwich GD, Burdick JA. Recent advances in hyaluronic acid hydrogels for biomedical applications. Curr Opin Biotechnol. 2016;40:35–40. [DOI] [PubMed] [Google Scholar]
- 102.Baek J, et al. Facile strategy involving low-temperature chemical cross-linking to enhance the physical and biological properties of hyaluronic acid hydrogel. Carbohydr Polym. 2018;202:545–53. [DOI] [PubMed] [Google Scholar]
- 103.Ardean C, et al. Factors influencing the antibacterial activity of Chitosan and Chitosan modified by functionalization. Int J Mol Sci. 2021;22(14):7449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Luanda A, Badalamoole V. Past, present and future of biomedical applications of dextran-based hydrogels: A review. Int J Biol Macromol. 2023;228:794–807. [DOI] [PubMed] [Google Scholar]
- 105.Sun G, Mao JJ. Engineering Dextran-Based scaffolds for drug delivery and tissue repair. Nanomedicine. 2012;7(11):1771–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Roberts JJ, et al. In situ formation of poly(vinyl alcohol)–heparin hydrogels for mild encapsulation and prolonged release of basic fibroblast growth factor and vascular endothelial growth factor. J Tissue Eng. 2016;7:2041731416677132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liang Y, Kiick KL. Heparin-functionalized polymeric biomaterials in tissue engineering and drug delivery applications. Acta Biomater. 2014;10(4):1588–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.He C, et al. Heparin-based and heparin-inspired hydrogels: size-effect, gelation and biomedical applications. J Mater Chem B. 2019;7(8):1186–208. [DOI] [PubMed] [Google Scholar]
- 109.Chen C, Xi Y, Weng Y. Recent advances in Cellulose-Based hydrogels for tissue engineering applications. Polymers. 2022;14(16):3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Singh P, et al. 3D-printed cellulose nanocrystals and gelatin scaffolds with bioactive cues for regenerative medicine: advancing biomedical applications. Int J Biol Macromol. 2024;278:134402. [DOI] [PubMed] [Google Scholar]
- 111.Mokhtari H, et al. Recent advances in Chemically-Modified and hybrid Carrageenan-Based platforms for drug delivery, wound healing, and tissue engineering. Polymers. 2021;13(11):1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu J, et al. Review for carrageenan-based pharmaceutical biomaterials: favourable physical features versus adverse biological effects. Carbohydr Polym. 2015;121:27–36. [DOI] [PubMed] [Google Scholar]
- 113.Elangwe CN, et al. Evaluation of composition effects on the tissue-adhesive, mechanical and physical properties of physically crosslinked hydrogels based on Chitosan and Pullulan for wound healing applications. Int J Biol Macromol. 2024;276:133857. [DOI] [PubMed] [Google Scholar]
- 114.Zarrintaj P, et al. Agarose-based biomaterials for tissue engineering. Carbohydr Polym. 2018;187:66–84. [DOI] [PubMed] [Google Scholar]
- 115.Ingavle GC, Gehrke SH, Detamore MS. The bioactivity of agarose–PEGDA interpenetrating network hydrogels with covalently immobilized RGD peptides and physically entrapped Aggrecan. Biomaterials. 2014;35(11):3558–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Su T, et al. Mussel-inspired agarose hydrogel scaffolds for skin tissue engineering. Bioactive Mater. 2021;6(3):579–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wu X, et al. Fully physically crosslinked pectin-based hydrogel with high stretchability and toughness for biomedical application. Int J Biol Macromol. 2020;149:707–16. [DOI] [PubMed] [Google Scholar]
- 118.Li D-q, et al. Pectin in biomedical and drug delivery applications: A review. Int J Biol Macromol. 2021;185:49–65. [DOI] [PubMed] [Google Scholar]
- 119.Qamruzzaman M, Ahmed F, Mondal MIH. An overview on starch-based sustainable hydrogels: potential applications and aspects. J Polym Environ. 2022;30(1):19–50. [Google Scholar]
- 120.Lee C-S, Hwang HS. Starch-based hydrogels as a drug delivery system in biomedical applications. Gels. 2023;9(12):951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sahoo DR, Biswal T. Alginate and its application to tissue engineering. SN Appl Sci. 2021;3(1):30. [Google Scholar]
- 122.Hernández-González AC, Téllez-Jurado L, Rodríguez-Lorenzo LM. Alginate hydrogels for bone tissue engineering, from injectables to bioprinting: A review. Carbohydr Polym. 2020;229:115514. [DOI] [PubMed] [Google Scholar]
- 123.Singh S, Rai AK, Tewari RP. Recent advancement in hyaluronic acid-based hydrogel for biomedical engineering application: A mini-review. Mater Today: Proc. 2023;78:138–44. [Google Scholar]
- 124.Rashki Ghaleno L et al. Exploring the Role of Hyaluronic Acid in Reproductive Biology and Beyond: Applications in Assisted Reproduction and Tissue Engineering. Advanced biology, 2024: p. 2300621. [DOI] [PubMed]
- 125.Liu F, et al. Hyaluronic acid hydrogel integrated with mesenchymal stem cell-secretome to treat endometrial injury in a rat model of Asherman’s syndrome. Adv Healthc Mater. 2019;8(14):1900411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Berger J, et al. Structure and interactions in covalently and ionically crosslinked Chitosan hydrogels for biomedical applications. Eur J Pharm Biopharm. 2004;57(1):19–34. [DOI] [PubMed] [Google Scholar]
- 127.Baytas SN, Linhardt RJ. Advances in the Preparation and synthesis of heparin and related products. Drug Discovery Today. 2020;25(12):2095–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Atala A. Tissue engineering of reproductive tissues and organs. Fertil Steril. 2012;98(1):21–9. [DOI] [PubMed] [Google Scholar]
- 129.Lin Y, et al. Synergistic regenerative therapy of thin endometrium by human placenta-derived mesenchymal stem cells encapsulated within hyaluronic acid hydrogels. Stem Cell Res Ther. 2022;13(1):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tavana S, et al. Hyaluronic acid-based hydrogel scaffold without angiogenic growth factors enhances ovarian tissue function after autotransplantation in rats. Biomed Mater. 2016;11(5):055006. [DOI] [PubMed] [Google Scholar]
- 131.Zhang D, et al. Functionalized human umbilical cord mesenchymal stem cells and injectable HA/Gel hydrogel synergy in endometrial repair and fertility recovery. Acta Biomater. 2023;167:205–18. [DOI] [PubMed] [Google Scholar]
- 132.Khunmanee S, et al. Thiol-yne click crosslink hyaluronic acid/chitosan hydrogel for three-dimensional in vitro follicle development. Mater Today Bio. 2023;23:100867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Huang Y, et al. Sodium alginate-bioglass-encapsulated hAECs restore ovarian function in premature ovarian failure by stimulating angiogenic factor secretion. Stem Cell Res Ther. 2021;12:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jamalzaei P, et al. Applicability of hyaluronic acid-alginate hydrogel and ovarian cells for in vitro development of mouse preantral follicles. Cell J (Yakhteh). 2020;22(Suppl 1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lv H, et al. Injectable, degradable, and mechanically adaptive hydrogel induced by L-serine and allyl-functionalized Chitosan with platelet-rich plasma for treating intrauterine adhesions. Acta Biomater. 2024;184:144–55. [DOI] [PubMed] [Google Scholar]
- 136.Hassani F, et al. Chitosan hydrogel supports integrity of ovarian follicles during in vitro culture: a preliminary of a novel biomaterial for three dimensional culture of ovarian follicles. Cell J (Yakhteh). 2019;21(4):479–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nason-Tomaszewski CE, et al. Extracellular matrix-templating fibrous hydrogels promote ovarian tissue remodeling and oocyte growth. Bioactive Mater. 2024;32:292–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cai H, et al. Local delivery of silk-cellulose incorporated with stromal cell-derived factor-1α functionally improves the uterus repair. Tissue Eng Part A. 2019;25(21–22):1514–26. [DOI] [PubMed] [Google Scholar]
- 139.Müller SA, et al. A hydrogel for adhesion prevention: characterization and efficacy study in a rabbit uterus model. Eur J Obstet Gynecol Reproductive Biology. 2011;158(1):67–71. [DOI] [PubMed] [Google Scholar]
- 140.Hill-West JL, et al. Efficacy of a resorbable hydrogel barrier, oxidized regenerated cellulose, and hyaluronic acid in the prevention of ovarian adhesions in a rabbit model. Fertil Steril. 1994;62(3):630–4. [DOI] [PubMed] [Google Scholar]
- 141.Ul-Islam M, et al. Development of three-dimensional bacterial cellulose/chitosan scaffolds: analysis of cell-scaffold interaction for potential application in the diagnosis of ovarian cancer. Int J Biol Macromol. 2019;137:1050–9. [DOI] [PubMed] [Google Scholar]
- 142.Liu F, Duan G, Yang H. Recent advances in exploiting carrageenans as a versatile functional material for promising biomedical applications. Int J Biol Macromol. 2023;235:123787. [DOI] [PubMed] [Google Scholar]
- 143.Wang L et al. Dual-Crosslinked bioactive hydrogel scaffold for accelerated repair of genital tract defect. Adv Funct Mater, 2024: p. 2405966.
- 144.Sánchez-Sánchez M-P, et al. Chitosan and kappa-carrageenan vaginal acyclovir formulations for prevention of genital herpes. In vitro and ex vivo evaluation. Mar Drugs. 2015;13(9):5976–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bang S, et al. Preventing postoperative tissue adhesion using injectable carboxymethyl cellulose-pullulan hydrogels. Int J Biol Macromol. 2017;105:886–93. [DOI] [PubMed] [Google Scholar]
- 146.An JM, et al. Carboxymethyl cellulose, pluronic, and pullulan-based compositions efficiently enhance antiadhesion and tissue regeneration properties without using any drug molecules. ACS Appl Mater Interfaces. 2021;13(14):15992–6006. [DOI] [PubMed] [Google Scholar]
- 147.Bang S, et al. Injectable Pullulan hydrogel for the prevention of postoperative tissue adhesion. Int J Biol Macromol. 2016;87:155–62. [DOI] [PubMed] [Google Scholar]
- 148.Park JE, et al. In vitro maturation on ovarian granulosa cells encapsulated in agarose matrix improves developmental competence of Porcine oocytes. Theriogenology. 2021;164:42–50. [DOI] [PubMed] [Google Scholar]
- 149.Alkali IM, et al. Culture of vitrified bovine ovarian tissue on agarose gel inserts maintains follicle integrity. Reproduction. 2023;166(5):299–310. [DOI] [PubMed] [Google Scholar]
- 150.Jabari A, et al. In vitro complete differentiation of human spermatogonial stem cells to morphologic spermatozoa using a hybrid hydrogel of agarose and laminin. Int J Biol Macromol. 2023;235:123801. [DOI] [PubMed] [Google Scholar]
- 151.Gholami K, et al. The air-liquid interface culture of the mechanically isolated seminiferous tubules embedded in agarose or alginate improves in vitro spermatogenesis at the expense of attenuating their integrity. Vitro Cell Dev Biology-Animal. 2020;56:p261–270. [DOI] [PubMed] [Google Scholar]
- 152.Wu Y, et al. E2-Loaded microcapsules and bone Marrow–Derived mesenchymal stem cells with injectable scaffolds for endometrial regeneration application. Tissue Eng Part A. 2024;30(3–4):115–30. [DOI] [PubMed] [Google Scholar]
- 153.Chandran S, et al. Potential use of drug loaded nano composite pectin scaffolds for the treatment of ovarian cancer. Curr Drug Deliv. 2013;10(3):326–35. [DOI] [PubMed] [Google Scholar]
- 154.Osmałek T, et al. Recent advances in polymer-based vaginal drug delivery systems. Pharmaceutics. 2021;13(6):884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gök MK, et al. Development of starch based mucoadhesive vaginal drug delivery systems for application in veterinary medicine. Carbohydr Polym. 2016;136:63–70. [DOI] [PubMed] [Google Scholar]
- 156.Ahmad A. Safety and toxicity implications of multifunctional drug delivery nanocarriers on reproductive systems in vitro and in vivo. Front Toxicol. 2022;4:895667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chon SJ, Umair Z, Yoon M-S. Premature ovarian insufficiency: past, present, and future. Front Cell Dev Biology. 2021;9:672890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sánchez-Ajofrín I, et al. A biomimetic Follicle‐Based design for engineering reproductive technologies. Adv Funct Mater. 2024;34(4):2310787. [Google Scholar]
- 159.Xiang D, et al. Advances in the applications of polymer biomaterials for in vitro follicle culture. Biomed Pharmacother. 2021;140:111422. [DOI] [PubMed] [Google Scholar]
- 160.Ghahremani-Nasab M, et al. Premature ovarian failure and tissue engineering. J Cell Physiol. 2020;235(5):4217–26. [DOI] [PubMed] [Google Scholar]
- 161.Igboeli P, et al. Intraovarian injection of autologous human mesenchymal stem cells increases Estrogen production and reduces menopausal symptoms in women with premature ovarian failure: two case reports and a review of the literature. J Med Case Rep. 2020;14(1):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wang M-Y, et al. Adult stem cell therapy for premature ovarian failure: from bench to bedside. Tissue Eng Part B: Reviews. 2022;28(1):63–78. [DOI] [PubMed] [Google Scholar]
- 163.Ulin M, et al. Human mesenchymal stem cell therapy and other novel treatment approaches for premature ovarian insufficiency. Reproductive Sci. 2021;28(6):1688–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lai D, et al. Human endometrial mesenchymal stem cells restore ovarian function through improving the renewal of germline stem cells in a mouse model of premature ovarian failure. J Translational Med. 2015;13(1):155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Fu Y-X, et al. Human mesenchymal stem cell treatment of premature ovarian failure: new challenges and opportunities. Stem Cell Res Ther. 2021;12:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhang D, et al. Applications of hydrogels in premature ovarian failure and intrauterine adhesion. Front Mater. 2022;9:942957. [Google Scholar]
- 167.Kuchakzadeh F, Ai J, Ebrahimi-Barough S. Tissue engineering and stem cell-based therapeutic strategies for premature ovarian insufficiency. Regenerative Therapy. 2024;25:10–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hu B, et al. Prospects for fertility preservation: the ovarian organ function reconstruction techniques for oogenesis, growth and maturation in vitro. Front Physiol. 2023;14:1177443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Francés-Herrero E, et al. Future challenges and opportunities of extracellular matrix hydrogels in female reproductive medicine. Int J Mol Sci. 2022;23(7):3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zarnaghi MR, et al. Assessment of apoptosis and oxidative stress in cryopreserved ovary after grafting in fibrin-alginate scaffold with endothelial cells and melatonin in Wistar rats. J Gynecol Obstet Hum Reprod. 2024;102828:p. [DOI] [PubMed] [Google Scholar]
- 171.Francés-Herrero E, et al. Advances of xenogeneic ovarian extracellular matrix hydrogels for in vitro follicle development and oocyte maturation. Biomaterials Adv. 2023;151:213480. [DOI] [PubMed] [Google Scholar]
- 172.Francés-Herrero E et al. Growth factor-loaded ovarian extracellular matrix hydrogels promote in vivo ovarian niche regeneration and enhance fertility in premature ovarian insufficiency preclinical models. Acta Biomater, 2024. [DOI] [PubMed]
- 173.Francés-Herrero E, et al. Growth factor-loaded ovarian extracellular matrix hydrogels promote in vivo ovarian niche regeneration and enhance fertility in premature ovarian insufficiency preclinical models. Acta Biomater. 2024;186:125–40. [DOI] [PubMed] [Google Scholar]
- 174.Rios PD, et al. Retrievable hydrogels for ovarian follicle transplantation and oocyte collection. Biotechnol Bioeng. 2018;115(8):2075–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chiti MC, et al. Ovarian extracellular matrix-based hydrogel for human ovarian follicle survival in vivo: A pilot work. J Biomedical Mater Res Part B: Appl Biomaterials. 2022;110(5):1012–22. [DOI] [PubMed] [Google Scholar]
- 176.Park KE, et al. Effects of alginate hydrogels on in vitro maturation outcome of mouse preantral follicles. Tissue Eng Regenerative Med. 2012;9:170–4. [Google Scholar]
- 177.Brito I, et al. Alginate hydrogel matrix stiffness influences the in vitro development of caprine preantral follicles. Mol Reprod Dev. 2014;81(7):636–45. [DOI] [PubMed] [Google Scholar]
- 178.Sánchez-Ajofrín I, et al. A biomimetic Follicle-Based design for engineering reproductive technologies. Adv Funct Mater. 2024;34(4):2310787. [Google Scholar]
- 179.Mihajlovic M, et al. 3D culture of ovarian follicles in granular and nanofibrillar hydrogels. Biomaterials Adv. 2024;164:213987. [DOI] [PubMed] [Google Scholar]
- 180.Zhao X, et al. A novel Three-Dimensional follicle culture system decreases oxidative stress and promotes the prolonged culture of human granulosa cells. ACS Appl Mater Interfaces. 2023;15(12):15084–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Van Eyck A-S, et al. Electron paramagnetic resonance as a tool to evaluate human ovarian tissue reoxygenation after xenografting. Fertil Steril. 2009;92(1):374–81. [DOI] [PubMed] [Google Scholar]
- 182.Lee J, et al. Ovarian injury during cryopreservation and transplantation in mice: a comparative study between cryoinjury and ischemic injury. Hum Reprod. 2016;31(8):1827–37. [DOI] [PubMed] [Google Scholar]
- 183.Youm HW, et al. Transplantation of mouse ovarian tissue: comparison of the transplantation sites. Theriogenology. 2015;83(5):854–61. [DOI] [PubMed] [Google Scholar]
- 184.Park D, et al. Hyaluronic acid promotes angiogenesis by inducing RHAMM-TGFβ receptor interaction via CD44-PKCδ. Mol Cells. 2012;33(6):563–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Gao J, et al. Effect of local basic fibroblast growth factor and vascular endothelial growth factor on subcutaneously allotransplanted ovarian tissue in ovariectomized mice. PLoS ONE. 2015;10(7):e0134035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Huang Y, et al. Sodium alginate-bioglass-encapsulated hAECs restore ovarian function in premature ovarian failure by stimulating angiogenic factor secretion. Stem Cell Res Ther. 2021;12(1):223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sabry D, et al. An experimental model: intrauterine adhesion versus subendometrial fibrosis. Biomed Res. 2018;29(17):3311–8. [Google Scholar]
- 188.Patel SK, et al. Drug delivery strategies for management of women’s health issues in the upper genital tract. Adv Drug Deliv Rev. 2021;177:113955. [DOI] [PubMed] [Google Scholar]
- 189.Milcovich G et al. Recent advances in smart biotechnology: Hydrogels and nanocarriers for tailored bioactive molecules depot. 2017. 249: pp. 163–180. [DOI] [PubMed]
- 190.Yang H, et al. Multifunctional group mediated double cross-linked polysaccharide complex hydrogel for microenvironmental regulation and repair of endometrial injury. Chem Eng J. 2024;485:149843. [Google Scholar]
- 191.Ma J, et al. Recent trends in therapeutic strategies for repairing endometrial tissue in intrauterine adhesion. Biomaterials Res. 2021;25:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Fang Z, et al. Injectable self-assembled dual-crosslinked alginate/recombinant collagen-based hydrogel for endometrium regeneration. Int J Biol Macromol. 2023;236:123943. [DOI] [PubMed] [Google Scholar]
- 193.Xie X, et al. Injectable, stable, and biodegradable hydrogel with platelet-rich plasma induced by l-serine and sodium alginate for effective treatment of intrauterine adhesions. Int J Biol Macromol. 2024;270:132363. [DOI] [PubMed] [Google Scholar]
- 194.Nie N, et al. 3D bio-printed endometrial construct restores the full-thickness morphology and fertility of injured uterine endometrium. Acta Biomater. 2023;157:187–99. [DOI] [PubMed] [Google Scholar]
- 195.Ji W, et al. 3D Bioprinting a human iPSC-derived MSC-loaded scaffold for repair of the uterine endometrium. Acta Biomater. 2020;116:268–84. [DOI] [PubMed] [Google Scholar]
- 196.Cai Y, et al. Porous scaffolds from droplet microfluidics for prevention of intrauterine adhesion. Acta Biomater. 2019;84:222–30. [DOI] [PubMed] [Google Scholar]
- 197.Xin L, et al. In situ delivery of apoptotic bodies derived from mesenchymal stem cells via a hyaluronic acid hydrogel: a therapy for intrauterine adhesions. Bioactive Mater. 2022;12:107–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Lebovitz O, Orvieto R. Treating patients with thin endometrium–an ongoing challenge. Gynecol Endocrinol. 2014;30(6):409–14. [DOI] [PubMed] [Google Scholar]
- 199.Kim YY, et al. Synergistic regenerative effects of functionalized endometrial stromal cells with hyaluronic acid hydrogel in a murine model of uterine damage. Acta Biomater. 2019;89:139–51. [DOI] [PubMed] [Google Scholar]
- 200.Zhang SS, et al. Using 17β-estradiol heparin‐poloxamer thermosensitive hydrogel to enhance the endometrial regeneration and functional recovery of intrauterine adhesions in a rat model. FASEB J. 2020;34(1):446–57. [DOI] [PubMed] [Google Scholar]
- 201.Zhou Q, et al. Self-assembled biocompatible heparin-based supramolecular hydrogel for doxorubicin delivery. Carbohydr Res. 2022;511:108464. [DOI] [PubMed] [Google Scholar]
- 202.Kim T, et al. A randomized, multi-center, clinical trial to assess the efficacy and safety of alginate carboxymethylcellulose hyaluronic acid compared to carboxymethylcellulose hyaluronic acid to prevent postoperative intrauterine adhesion. J Minim Invasive Gynecol. 2012;19(6):731–6. [DOI] [PubMed] [Google Scholar]
- 203.Vatanatara J, et al. Alginate carboxymethylcellulose hyaluronic acid for preventing intrauterine adhesion after vacuum aspiration for first-trimester abortion: a prospective, randomized controlled trial. J Gynecologic Surg. 2021;37(5):402–7. [Google Scholar]
- 204.Soliman GM, Fetih G, Abbas AM. Thermosensitive bioadhesive gels for the vaginal delivery of sildenafil citrate: in vitro characterization and clinical evaluation in women using clomiphene citrate for induction of ovulation. Drug Dev Ind Pharm. 2017;43(3):399–408. [DOI] [PubMed] [Google Scholar]
- 205.Zhang H, et al. Chitosan combined with intrauterine device prevents intrauterine adhesions after hysteroscopic adhesiolysis: A target trial emulation study. J Obstet Gynecol Res. 2023;49(6):1571–8. [DOI] [PubMed] [Google Scholar]
- 206.Wang Y-Q, et al. Comparison of autocross-linked hyaluronic acid gel and intrauterine device for preventing intrauterine adhesions in infertile patients: a randomized clinical trial. Gynecol Minim Invasive Therapy. 2020;9(2):74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Bhoi NR et al. Effect of Hyaluronic Acid-Containing Transfer Media (EmbryoGlue®) on the Live Birth Rate in Frozen Thawed Embryo Transfer Cycles. Cureus, 2024. 16(1). [DOI] [PMC free article] [PubMed]
- 208.Tüttelmann F, Ruckert C, Röpke A. Disorders of spermatogenesis: perspectives for novel genetic diagnostics after 20 years of unchanged routine. Med Genet. 2018;30(1):12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Esteves SC. Male infertility due to spermatogenic failure: current management and future perspectives. Anim Reprod. 2015;12:62–80. [Google Scholar]
- 210.Yoshimasa Y, Maruyama T. Bioengineering of the uterus. Reproductive Sci. 2021;28(6):1596–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Xu X, Lai Y, Hua Z-C. Apoptosis and apoptotic body: disease message and therapeutic target potentials. Biosci Rep. 2019;39(1):BSR20180992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zhou M, et al. Apoptotic bodies for advanced drug delivery and therapy. J Controlled Release. 2022;351:394–406. [DOI] [PubMed] [Google Scholar]
- 213.Jahanbani Y, et al. Stem cells technology as a platform for generating reproductive system organoids and treatment of infertility-related diseases. Cell Biol Int. 2022;46(4):512–22. [DOI] [PubMed] [Google Scholar]
- 214.Ghasemi D, et al. Differentiation of human endometrial stem cells encapsulated in alginate hydrogel into oocyte-like cells. Volume 13. BioImpacts: BI; 2023. p. 229. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Chung JT, Lau CML, Chau Y. The effect of polysaccharide-based hydrogels on the response of antigen-presenting cell lines to immunomodulators. Biomaterials Sci. 2021;9(19):6542–54. [DOI] [PubMed] [Google Scholar]
- 216.Tan J, et al. Development of alginate-based hydrogels: crosslinking strategies and biomedical applications. Int J Biol Macromol. 2023;239:124275. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.