Abstract
Mitochondrial function relies on the coordinated interactions between genes in the mitochondrial DNA and nuclear genomes. Imperfect interactions following mitonuclear incompatibility may lead to reduced fitness. Mitochondrial DNA introgressions across species and populations are common and well documented. Various strategies may be expected to reconcile mitonuclear incompatibility in hybrids or admixed individuals. African admixed cattle (Bos taurus × B. indicus) show sex-biased admixture, with taurine (B. taurus) mitochondrial DNA and a nuclear genome predominantly of humped zebu (B. indicus). Here, we leveraged local ancestry inference approaches to identify the ancestry and distribution patterns of nuclear functional genes associated with the mitochondrial oxidative phosphorylation process in the genomes of African admixed cattle. We show that most of the nuclear genes involved in mitonuclear interactions are under selection and of humped zebu ancestry. Variations in mitochondrial DNA copy number may have contributed to the recovery of optimal mitochondrial function following admixture with the regulation of gene expression, alleviating or nullifying mitochondrial dysfunction. Interestingly, some nuclear mitochondrial genes with enrichment in taurine ancestry may have originated from ancient African aurochs (B. primigenius africanus) introgression. They may have contributed to the local adaptation of African cattle to pathogen burdens. Our study provides further support and new evidence showing that the successful settlement of cattle across the continent was a complex mechanism involving adaptive introgression, mitochondrial DNA copy number variation, regulation of gene expression, and selection of ancestral mitochondria-related genes.
Keywords: mitonuclear interactions, mitonuclear DNA discordance, mtDNA copy numbers, African cattle, purifying selection, admixture
Introduction
The biological function of the mitochondria is maintained by the coordination of mitonuclear interplay through the interactions between mitochondrial DNA (mtDNA) proteins, encoded by 13 genes, and nuclear-coded proteins expressed from ∼1,200 genes (Havird et al. 2019; Mottis et al. 2019). The mitonuclear compatibility illustrates that the products of mitochondrial and nuclear genes must be physically and functionally integrated (i.e. mitonuclear concordance) to empower the respiratory complexes of the electron transport system (ETS) and to enable the transcription and translation of mitochondrial genes as well as the replication of mtDNA (Rand et al. 2004; Quiros et al. 2016; Hill 2019a; Lynch 2023). When two evolutionary lineages hybridize or admix with a sex bias, mismatched mitochondrial and nuclear genotypes may disrupt mitonuclear interactions, resulting in varying levels of mitonuclear DNA discordance (Toews and Brelsford 2012; Bonnet et al. 2017; Zaidi and Makova 2019). Mitonuclear DNA discordance may disrupt the physical and functional integrity of mitonuclear proteins and decrease the electron transport capacity of ETS complexes (Hill 2019a, 2020), which cause an increase in free radicals, generate higher levels of reactive oxygen species, reduce ATP generation, and impair oxidative respiration processes, leading to mitochondrial dysfunction (Ma et al. 2016; Havird et al. 2019; Hill 2019a). Furthermore, mitonuclear DNA discordance can also slow mtDNA replication efficiency (Bonnet et al. 2017) or trigger the re-fusion of mitochondria to alleviate the dysfunction (MacVicar and Langer 2016; Prashar et al. 2024; Tabara et al. 2024), ultimately decreasing mtDNA copy numbers (mtDNA-CN) (Ellison and Burton 2008, 2010; Ciesielski et al. 2016; Bailey and Doherty 2017) and total respiratory capacity (Hill 2019a). In addition, adverse effects on fertility, survivability, development, and environmental adaptation with reduced hybrid fitness may be observed (Lane 2011; Wolff et al. 2014; Moran et al. 2024), as explained by the Bateson–Dobzhansky–Muller (BDM) model (Cutter 2012; Havird et al. 2019; Koch et al. 2021). Several potential mechanisms to mitigate mitonuclear incompatibilities have been proposed (Sloan et al. 2017; Hill 2019a, 2020).
The mitonuclear coevolution hypothesis indicates co-occurrence and interactions between mitochondrial and nuclear genomes with fitness in eukaryotes influenced by reciprocal evolutionary changes driven by selection influence fitness in eukaryotes (Rand et al. 2004; Sloan et al. 2018; Hill 2019a; Weaver et al. 2022). Selection in nuclear genes involved in mitochondrial function can reduce mitonuclear DNA discordance, thereby maintaining mitonuclear compatibility (Havird and Sloan 2016; Barreto et al. 2018; Zaidi and Makova 2019; Hill 2020; Pereira et al. 2021; Weaver et al. 2022). Hybrids of intertidal copepod Tigriopus californicus that exhibit enrichment of nuclear and mtDNA alleles from the same maternal parental population show higher fitness (Healy and Burton 2020). The enrichment of nuclear and mtDNA alleles from the same maternal sources aligns with the BDM model (Hill 2019b; Healy and Burton 2020; Kwon et al. 2022). Nevertheless, when mitonuclear mismatches persist, some compensation mechanisms have been proposed to maintain mitochondrial function (Wolff et al. 2014; Hill 2016, 2020). In the mtDNA adaptive introgression mechanism, the exotic mtDNA allele may harbor a lower mutational load or confer greater adaptability to the environment resulting in increased fitness in hybrids (Deremiens et al. 2015; Bonnet et al. 2017; Sloan et al. 2017; Hill 2019a). The alternative, the nuclear compensation mechanism (Barreto et al. 2018; Hill 2019a, 2020; Lynch 2023; Tao et al. 2024), suggests that novel nuclear-encoded amino acid sequences or proteins may reverse or alleviate mitonuclear dysfunction (Hughes 2013; Rong et al. 2021). The evolution of the nuclear compensation mechanism has been supported by empirical investigations in several species (Barreto and Burton 2013; Barreto et al. 2018) as well as simulation modeling (Princepe and de Aguiar 2024). However, the specific mechanisms and factors driving the nuclear compensation mechanism remain unclear and debated.
Indigenous African humped cattle (e.g. zebu, sanga, and zenga) are characterized by a complex history of hybridization with multiple waves of introgression and migration (Hanotte et al. 2002; Reist-Marti et al. 2003; Kim et al. 2020). The two different cattle lineages, humpless taurine cattle (B. taurus) and humped zebu cattle (B. indicus) originated from distinct subspecies of aurochs (B. primigenius), which diverged approximately 0.2 to 1 million years before present (YBP) (Loftus et al. 1994; Hanotte et al. 2000; Chen et al. 2018, 2023; Ginja et al. 2023). The humpless taurine were domesticated around 10,000 YBP in the Fertile Crescent, followed by the domestication of the humped zebu approximately 8,000 YBP in the Indus Valley (Loftus et al. 1994; Chen et al. 2010; Verdugo et al. 2019). The humpless taurine cattle were first introduced into North Africa circa 7,000 YBP, and they subsequently spread to West and East Africa circa 4,000 to 3,000 YBP (Gifford-Gonzalez and Hanotte 2011; Kim et al. 2020). The main introduction of the humped zebu cattle into Africa can be traced to at least 1,400 YBP (Hanotte et al. 2002; Linseele et al. 2016). The mtDNA of all African cattle is today exclusively taurine, while the Y chromosome and autosomes of African humped cattle are predominantly of zebu origin (Bonfiglio et al. 2012; Verdugo et al. 2019; Kim et al. 2020; Mauki et al. 2021; Kwon et al. 2022; Ward et al. 2022; Chen et al. 2023). A male sex-biased crossbreeding has been postulated to be at the root of this discrepancy between the mitochondrial and nuclear ancestries of the present-day African admixed cattle (Hanotte et al. 2000; Verdugo et al. 2019; Kwon et al. 2022; Chen et al. 2023).
Two recent population genomic studies have shown enrichment of taurine ancestry in some nuclear genes involved in mitonuclear interactions (Kwon et al. 2022; Ward et al. 2022), thus supporting the hypothesis of mitonuclear coevolution. These two studies did not investigate possible explanations for the predominant zebu ancestry in other autosomal regions involved in mitonuclear interplay. Herein, we dissect the evolutionary ancestries of functional mitonuclear genes in multiple African admixed cattle populations to evaluate the level of mitonuclear DNA discordance. We then investigate possible explanations for the presence or absence of ancestral mitonuclear compatibility and further uncover the underlying mechanisms of how African admixed cattle populations cope with mitonuclear incompatibilities.
Results
Identifying the Source Populations of the African B. taurus × B. indicus Populations
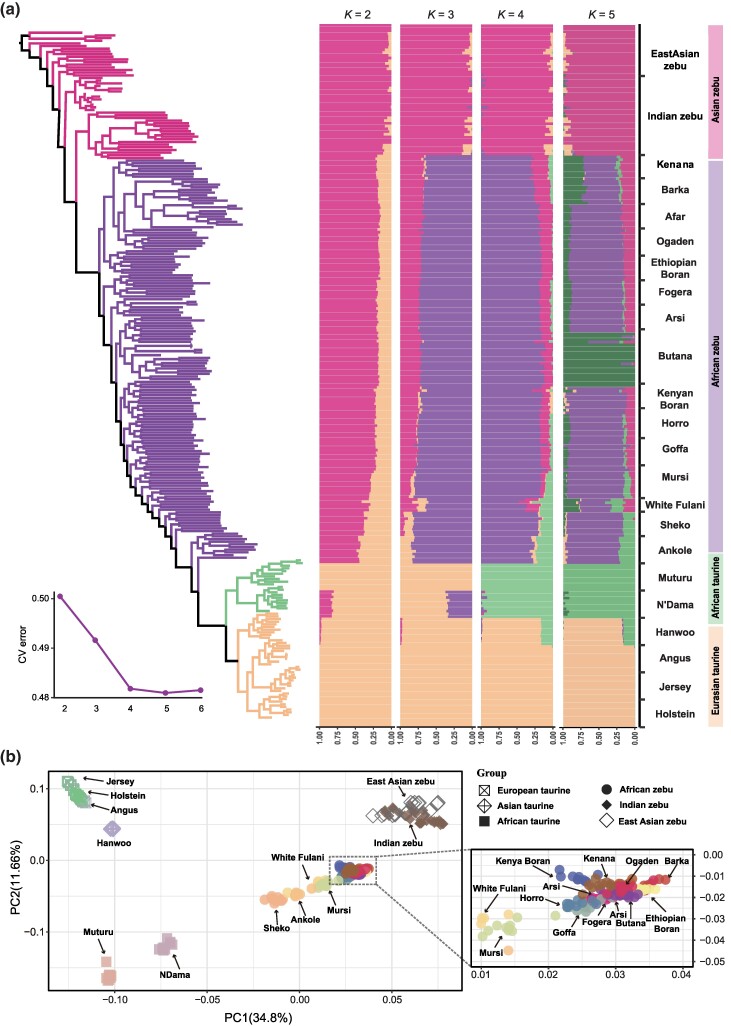
We conducted population genomic analysis using a core dataset of 258 cattle genomes (Additional file 1: supplementary table S1, Supplementary Material online). Unsupervised clustering depicted the admixed patterns (B. taurus × B. indicus) of the studied African cattle predominantly from Asian zebu ancestry. This result was supported by principal component analysis (PCA) and maximum likelihood (ML) tree (Fig. 1). The studied African cattle populations also show a taurine background with a close genetic affinity to African than Eurasian taurine (K = 4 in Fig. 1a), consistent with previous findings (Kim et al. 2020; Ward et al. 2022). We, therefore, assigned African taurine and Asian zebu as ancestral donor populations for the African admixed cattle populations. The local ancestry inference indicated that approximately 25% of the nuclear genome (disregarding sex chromosomes) of the 15 African admixed breeds (n = 150 animals) is of taurine ancestry (Additional file 2: supplementary fig. S1, Supplementary Material online). All the mitochondrial genomes of African zebu and taurine stemmed from taurine (Additional file 1: supplementary table S1, Supplementary Material online). The African mtDNAs were assigned to taurine sub-haplogroup T1, except for one Arsi sample that belonged to sub-haplogroup T2. We calculated the ratio of nonsynonymous to synonymous substitutions (ω = dN/dS) for 13 mitochondrial genes of the oxidative phosphorylation system (OXPHOS) and only CYTB mt-gene showed ω > 1 in the branch of African cattle mtDNAs (Additional file 1: supplementary table S2, Supplementary Material online). However, the likelihood ratio test (LRT < 3.8415) was not significant (P > 0.05, Chi-square test), failing to support positive selection on African cattle mtDNA sequences (Ward et al. 2022).
Fig. 1.
Identifying potential donor populations of African admixed cattle. a) Phylogeny, population admixture analysis. b) PCA. After pruning for linkage disequilibrium, a total of 1,482,502 SNPs from 258 samples were analyzed. K indicates the number of ancestry components assumed in the ADMIXTURE analysis. The cross-validation error was substantially reduced at K = 4.
Characterizing Ancestries of Mitonuclear Genes
Four gene subsets were analyzed (Fig. 2a): (i) all nuclear genes associated with mitochondrial genome interplay (mitonuclear genes; n = 1,064) from MitoCarta 3.0 (Rath et al. 2021); (ii) genes of the oxidative phosphorylation system (OXPHOS genes; n = 149), which are directly involved in the assembly of the five OXPHOS complexes as organized by Signes and Fernandez-Vizarra (2018); (iii) nuclear genes with function related to mtDNA replication and transcription machinery, ribosomal functions, and OXPHOS complexes (high-mito genes; n = 144), as defined by Sloan et al. (2015); and (iv) mitonuclear interactions selected candidate genes (selective genes; n = 21), which are involved in the integrity of the cellular respiration system as reported by Kwon et al. (2022). We observed a highly significant decrease (P < 0.001) in nucleotide diversity (π) of the four mitochondria-related gene subsets, compared with the other nuclear coding genes and genome-wide (Fig. 2b and Additional file 2: supplementary fig. S2a, Supplementary Material online), indicating that these mitochondria-related genes are under strong selection pressure. Compared with the genome-wide background, the four gene sets showed significantly higher genetic differentiation (P < 0.001) between the two ancestral donor populations (i.e. Asian zebu and African taurine) (Fig. 2c), supporting the hypothesis that mitonuclear genes evolve at a faster rate than nonmitonuclear genes (Havird and Sloan 2016; Hill 2019a, 2020; Piccinini et al. 2021; Weaver et al. 2022). Consequently, the male-biased backcross breeding scheme introducing zebu nuclear ancestry but maintaining taurine mtDNA (Fig. 2a) increases mitonuclear DNA discordance in African admixed cattle populations.
Fig. 2.
Human-mediated admixture shaped the interactions between heterologous mtDNA and nuclear genomes of African admixed cattle. a) The brief history of admixture and the pattern of mitonuclear interactions in African admixed cattle. African cattle represent an excellent model for studying the genomic mechanisms mitigating the effect of mitonuclear DNA discordance as well as for understanding the genome interplay under suboptimal mitonuclear combination. b) Deviations of nucleotide diversity (π) between four gene sets involved in mitonuclear interactions in African admixed cattle. c) Deviations of genetic differentiation for four gene sets involved in mitonuclear interactions between Asian zebu and African taurine. The mitonuclear DNA discordance may be triggered by admixture of Asian zebu and African taurine due to unique mitonuclear coevolution. Box plots of π in a 50 kb genomic window with a sliding window of 10 kb. Significance was evaluated by two-sided t-tests (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001; and ns means no statistical significance).
We assessed the ancestry of mitonuclear genes in the genomes of African admixed cattle populations with multiple approaches (Fdm, FST, RFmix, and Loter) that infer local ancestry. The results showed a significant enrichment (P < 0.01) in B. indicus ancestry at the subsets of mitonuclear, high-mito, and OXPHOS genes compared with the genome-wide (Fig. 3a and Additional file 2: supplementary figs. S3 and S4, Supplementary Material online). When compared with all the coding genes, generally under purifying selection, most genes involved in mitonuclear interactions exhibited a significant enrichment in zebu ancestry (Fig. 3a and Additional file 2: supplementary figs. S3 and S4, Supplementary Material online). Random re-samplings showed that the enrichment of zebu ancestry in subsets of mitonuclear genes could be the result of selection rather than genetic drift (Additional file 2: supplementary fig. S2b to d, Supplementary Material online). Selective genes (n = 21) reported by Kwon et al. (2022) exhibited a highly significant increase in taurine ancestry (P < 0.001). The mean proportion of taurine ancestry was below the average of 25% (range 0% to 23.6%) for nine genes (Fig. 3a and Additional file 2: supplementary figs. S2 and S5a, Supplementary Material online). The remaining genes showed slightly higher enrichment in taurine ancestry (>25%) (e.g. SLC30A6: 48.2%, TRAK2: 48.1%, and CASP8: 41.7%) (Additional file 2: supplementary fig. S5, Supplementary Material online). The results suggest that most genes involved in mitonuclear interactions with taurine ancestries are eliminated in the genomes of African admixed cattle through selection on the mitochondria-related genes (Fig. 2b). The distributions of Tajima's D for the four mitochondria-related gene subsets shift to the donor population of Asian zebu (Fig. 3b), which is in accordance with the patterns of selection shaping local ancestry combinations in the genome following hybridization (Schumer et al. 2018; Moran et al. 2021). A similar deviation of local ancestry was found in an extended dataset of 593 African admixed cattle genomes from East, Central, and West African populations (Fig. 3a and Additional file 2: supplementary figs. S6 to S11, Supplementary Material online) and 95 Asian admixed taurine genomes (Additional file 2: supplementary fig. S12c and d, Supplementary Material online).
Fig. 3.
Deviations of ancestry for genes involved in mitonuclear interactions in African admixed cattle. a) Deviations in African admixed cattle local ancestry across genes involved in mitonuclear interactions. The values of FST, FdM, and Loter were calculated using a 50 kb window size and a 10 kb step size. (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001; and ns means no statistical significance). b) Density plots of estimates of Tajima's D for gene sets of mitonuclear (n = 1,064), high-mito (n = 144), OXPHOS (n = 149), and selective genes (n = 21) in African admixed cattle. Significance was evaluated by two-sided t-tests.
Nuclear Genetic Control in mtDNA-CN in African Cattle
We estimated the number of mtDNA-CN in whole blood cells based on genome coverage. All African cattle have significantly (P < 0.001) lower taurine mtDNA-CN compared with Eurasian taurine (Additional file 2: supplementary fig. S13a, Supplementary Material online). Most of the African admixed cattle breeds had lower mtDNA-CN compared with the Asian zebu (Fig. 4a). Interestingly, when the taurine ancestry is <25% of the genome-wide in African cattle populations (n = 116), we observed a significant negative correlation between mtDNA-CN and the proportion of taurine ancestry (Fig. 4b and R = −0.21, P-value = 0.025). The mtDNA-CN was highest in Barka, Kenyan Boran, and Ogaden populations, which had the lowest taurine ancestry (the mean proportion of taurine ancestry is <16%). In these populations, mtDNA-CN levels were similar to the observed in Asian zebu (Fig. 4). This pattern of elevated mtDNA-CN levels coupled with increased mitonuclear DNA discordance was also observed in Asian admixed taurine cattle (Additional file 2: supplementary fig. S12e and f, Supplementary Material online). The genome-wide association study (GWAS) for mtDNA-CN in African admixed cattle (n = 116) identified 148 genome-wide significant (P < 1× 10−7) loci (Fig. 5 and Additional file 1: supplementary table S3, Supplementary Material online and Additional file 2: supplementary fig. S13e, Supplementary Material online). They cumulatively explained 85.9% of the variation in mtDNA-CN in African admixed cattle (Fig. 5 and Additional file 1: supplementary table S3, Supplementary Material online). These single nucleotide polymorphisms (SNPs) mapped to 71 annotated protein-coding genes (Additional file 1: supplementary table S3, Supplementary Material online), but only two, LYPLAL1 and HARS2, are included in the mitonuclear genes (n = 1,064) from MitoCarta 3.0 (Rath et al. 2021). Overall, these 71 genes are more enriched in zebu ancestry compared with the genome-wide and in the four subsets of the studied mitonuclear genes (Fig. 5b). Among these 71 genes, 14 are associated with mtDNA-CN, mitochondrial protein synthesis, ATP generation, and mitochondrial function (Fig. 5a and Additional file 1: supplementary table S4, Supplementary Material online). For instance, ANKHD1 and PHOSPHO1 were reported previously to regulate mtDNA-CN (Kitamata et al. 2019; Jiang et al. 2020). These 14 mtDNA-CN genes were present within signatures of selective sweeps in African admixed populations (Additional file 1: supplementary table S5, Supplementary Material online and Additional file 2: supplementary fig. S14, Supplementary Material online). Compared with the mitonuclear genes (n = 1,064) from MitoCarta 3.0 (Rath et al. 2021), 12 of these 14 mtDNA-CN genes showed a higher level of zebu ancestry (Fig. 5c).
Fig. 4.
Correlation between mtDNA-CN and the ratio of taurine ancestry in African cattle. a) The ratio of taurine ancestry and mtDNA-CN in cattle. The black solid lines represent the medians of each boxplot and serve as links. The dashed line indicates 25% level of genome-wide taurine ancestry (Additional file 2: supplementary fig. S1, Supplementary Material online). b) The mtDNA-CN was negatively correlated (R = −0.21, P = 0.025) with African taurine ancestry in the populations (n = 116, highlighted in red in figure a) with <25% genome-wide ancestry.
Fig. 5.
Genetic correlation for mtDNA-CN variation in African admixed cattle. a) GWAS was performed with GEMMA for mtDNA-CN variation in African admixed cattle taken as a quantitative trait that follows a normal distribution (see Additional file 2: supplementary fig. S13b, Supplementary Material online). The blood mtDNA-CN was highly heritable as indicated by the heritability of 96.7% based on the genomic relationship matrix. The dashed line indicates the significance threshold of 1 × 10−7. Among the 71 coding genes associated with mtDNA-CN, 14 candidate genes playing roles in mtDNA-CN regulation, mitochondrial protein synthesis, ATP generation, and mitochondrial function are indicated in the Manhattan plot. b) Deviations in taurine ancestry for the 71 coding genes associated with mtDNA-CN compared with the genome-wide or four subsets of mitonuclear genes. c) Deviations in taurine ancestry across 14 candidate genes involving in mitochondrial function, metabolism, assembly, and mtDNA-CN. Statistical significance was calculated by two-sided t-tests (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001; and ns means no significance).
Around 97% (143/148) of the candidate SNPs are found in noncoding regions and potentially perform regulatory functions (Additional file 1: supplementary table S3, Supplementary Material online). We characterized 21 cis-eQTLs (Additional file 1: supplementary table S6, Supplementary Material online) using Cattle-GTEx (Liu et al. 2022). Two cis-eQTLs rs133309482 and rs133946601 potentially regulated the expression of CD14 (Zang et al. 2010), HARS2 (Xu et al. 2021), and SLC35A4 (Chang 2022; Rocha et al. 2024), that are involved in mitochondrial function and mitonuclear interactions (Additional file 2: supplementary fig. S15a, Supplementary Material online). These results suggest that selection favored zebu ancestry while eliminating taurine ancestry in mtDNA-CN associated genes, thereby facilitating the maintenance of mtDNA-CN and mitochondrial function in African admixed cattle (Fig. 6 and Additional file 2: supplementary fig. S15b, Supplementary Material online).
Fig. 6.
Nuclear compensation reconciles mitonuclear incompatibility in African cattle. The sex-biased crossbreeding is postulated to explain the discrepancy between mitochondrial (B. taurus: 100%) and nuclear (B. indicus: 75%) ancestries of present-day African humped cattle. Ever-increasing mitonuclear DNA discordance is inevitable in African humped cattle resulting in mitonuclear incompatibilities. Accordingly, we hypothesized three scenarios: (i) The enrichment of taurine ancestry (i.e. the source of mtDNA) under selection reduces mitonuclear DNA discordance, known as the mitonuclear coevolution mechanism; (ii) Mitonuclear genes are highly conserved between taurine and zebu, and their admixture may not trigger mitonuclear incompatibility; and (iii) Evolution of nuclear genes may help reverse or alleviate mitonuclear incompatibility, known as nuclear compensation. Our results indicate that most functional genes involved in mitonuclear interactions exhibited significant enrichment in African taurine ancestry under strong selection, failing to support the mitonuclear coevolution. While enriched zebu ancestry increases mitonuclear DNA discordance, it facilitates the evolution of nuclear compensation. Regulations in gene expression ascribed to different activities of regulatory elements from parental genomes mainly generate lower levels of reactive oxygen species, decrease oxidative stress, alleviate mitochondrial damage, increase ATP generation, and maintain mtDNA-CN, and maintain mitonuclear interactions.
Selective Genes with Enrichment of Taurine Ancestry
We further investigated the 21 selective genes proposed to be enriched in taurine ancestry (Kwon et al. 2022). Indeed, some genes (e.g. CASP8 and TRAK2) exhibited significant enrichment in African taurine ancestry (Additional file 2: supplementary fig. S5, Supplementary Material online). These 21 selective genes generally showed higher genetic diversity compared with other mitochondrial-related gene datasets (Fig. 2b), implying some of them might have ancient genetic origins. We then screened for selective sweeps in the genomes of the taurine source populations (Muturu and N’Dama) (Fig. 7a) and found evidence that selection on some genes (e.g. CASP8 and TRAK2) likely preceded admixture with Asian zebu. The ML tree for the ∼2.2 Mb region containing CASP8 and TRAK2 (chr2: 87,864,908 to 90,470,906) showed a higher level of divergence in the lineages with African taurine ancestry than those with Eurasian taurine ancestry (Fig. 7b). We further included ancient DNA data including B. primigenius samples to trace the origin of this region. The African taurine ancestry lineages exhibited the strongest genetic affiliation to the African aurochs B. p. africanus Th7 from Morocco, while showing divergence from other non-African ancient aurochs and domestic cattle samples (Additional file 2: supplementary fig. S16a, Supplementary Material online and Additional file 1: supplementary table S7, Supplementary Material online). The ML tree positioned African aurochs B. p. africanus Th7 at the base of the evolutionary branch of African taurine ancestry lineage, suggesting potential introgression from ancient African aurochs (Additional file 2: supplementary fig. S16c, Supplementary Material online). Haplotype networks also revealed that the African taurine ancestry lineage shared the Hap 1 with African aurochs Th7 (Additional file 2: supplementary fig. S16b, Supplementary Material online). Moreover, we observed that the African taurine ancestry lineage shares more homozygous SNPs with African aurochs B. p. africanus Th7 (Fig. 7c) and displays higher allele frequency in the ∼2.2 Mb region containing CASP8 and TRAK2 (chr2: 87,864,908 to 90,470,906), compared with the other lineages (Additional file 1: supplementary table S7, Supplementary Material online). These results suggest that some selected genes with enrichment of African taurine ancestry in modern African cattle might be ascribed to the genetic legacy of adaptive introgression from ancient African aurochs.
Fig. 7.
Selective sweeps and phylogeny for genes with enrichment of taurine ancestry. a) Selective sweeps for the 21 genes in the African taurine breeds Muturu and N’Dama. The dashed line indicates the top 1% of all the likelihood ratio values. Only loci with likelihood values >500 are displayed. b) Archaic adaptive introgression in African cattle was indicated by ML tree in the genomic region of chr2: 87,864,908 to 90,470,906. c) Shared homozygous loci with African aurochs Th7 in the genomic region of chr2: 87,864,908 to 90,470,906. Orange and blue dots, along with connecting lines, represent African taurine and Eurasian taurine ancestry, respectively. Green dots and lines indicate loci shared with Th7.
Validation in East Asian Cattle
Similarly, we investigated the mitonuclear DNA discordance in East Asian cattle genomes for two admixed breeds, Qinchuan and Jiaxian Red cattle (Additional file 1: supplementary table S8, Supplementary Material online). We first focused on one of the samples with haplogroup I from Asian zebu, and found that the local ancestry inferred by FST and RFmix showed a significant enrichment (P < 0.05) in taurine ancestry (rather than the source of mtDNA) in the subsets of mitonuclear genes from MitoCarta 3.0 (Rath et al. 2021) and OXPHOS genes (Signes and Fernandez-Vizarra 2018) compared with the genome-wide (Additional file 2: supplementary fig. S12c and d, Supplementary Material online). Then, we noted the link between population structure and mtDNA-CN. Compared with Qinchuan cattle, Jiaxian Red cattle were heterogeneous genome-wide (Additional file 2: supplementary fig. S12a and b, Supplementary Material online) and in local ancestry distribution (Additional file 2: supplementary fig. S12e, Supplementary Material online), mirroring the more stringent breeding practice and management of Qinchuan cattle (Xia et al. 2021). The Qinchuan cattle had more mtDNA-CN than the Jiaxian Red cattle (Additional file 2: supplementary fig. S12f, Supplementary Material online), supporting mtDNA as a marker in the genetic improvement of cattle. In Jiaxian Red cattle, samples with mtDNA haplogroup T showed a significantly higher proportion (P < 0.01) of taurine ancestry and more (P < 0.05) mtDNA-CN compared with those with haplogroup I (Additional file 2: supplementary fig. S12e and f, Supplementary Material online). Preserving taurine ancestry increased mitonuclear DNA discordance but also likely increased mtDNA-CN. Therefore, the mitonuclear DNA discordance and mtDNA-CN variation in the admixed East Asian cattle genomes were in line with the patterns observed in African admixed cattle.
Discussion
Elimination of Taurine Ancestry Increases Mitonuclear DNA Discordance
Our results illustrated that most of the genes involved in mitonuclear interactions exhibited no enrichment in African taurine ancestry in the admixed B. taurus × B. indicus African cattle (Fig. 3a and Additional file 2: supplementary figs. S3 and S4, Supplementary Material online). A recent study reported that high-mito genes (n = 144) displayed taurine enrichment in three (East African Shorthorn Zebu, Nganda, and Keteku) out of the ten African admixed cattle analyzed (Ward et al. 2022). The same study emphasized the potential roles of DARS2 and MRPS33, two genes enriched in taurine ancestry, in mitonuclear interplay (Ward et al. 2022). However, our findings revealed no taurine ancestry enrichment for high-mito genes, including DARS2 and MRPS33 (Fig. 3a and Additional file 2: supplementary fig. S17, Supplementary Material online). These two genes were not reported by Kwon et al. (2022) to be enriched in taurine ancestry (Kwon et al. 2022). These three populations of Ward et al. (2022) were not part of the current or Kwon et al. (2022) study. Differences in the datasets and analysis strategies make it difficult to compare findings. The ancestry deviations observed by Ward et al. (2022) may be influenced by SNP ascertainment bias or the low density of SNPs on the BovineHD 777K SNP chip data compared with whole genome sequences (Albrechtsen et al. 2010). Given the Near East origin of the taurine mtDNA haplogroup T (Bonfiglio et al. 2012; Horsburgh et al. 2013; Chen et al. 2023), the spread of taurine mtDNA across Africa is unlikely to be linked to adaptive mitochondrial introgression (Mwai et al. 2015; Mei et al. 2018; Utsunomiya et al. 2019; Chen et al. 2023). Our results failed to support positive selection in taurine mtDNA of African admixed cattle (Additional file: supplementary table S2, Supplementary Material online), although more mtDNA data and functional evidence are required. Thus, African admixed cattle have faced and are facing the challenge of recovering or maintaining mitonuclear interactions under the inevitably extended mitonuclear DNA discordance.
Maintaining mtDNA-CN to Mitigate Mitonuclear Incompatibility
Our analysis revealed mtDNA-CN variation in relation to mitonuclear DNA discordance in African admixed cattle. The reduction in mtDNA-CN observed in African admixed cattle, compared with Eurasian cattle populations (Fig. 4 and Additional file 2: supplementary fig. S13a, Supplementary Material online), supports the fact that increased mitonuclear DNA discordance may disrupt co-adapted mitonuclear interactions (Rand et al. 2004; Hill 2019a). Similar patterns have been observed in human admixed populations from the Americas, where mtDNA-CN decreases as mitonuclear DNA discordance increases (Zaidi and Makova 2019). Surprisingly, we also observed that mtDNA-CN of African admixed breeds increased with a decrease in African taurine ancestry (Fig. 4). The mtDNA-CN associated genes identified in GWAS were enriched in zebu ancestry (Fig. 5b and c) compared with the genome-wide background and were under selection (Additional file 1: supplementary table S8, Supplementary Material online). These observations led us to hypothesize that, to counteract the negative fitness effects of mitonuclear incompatibility caused by increased mitonuclear DNA discordance, selection on genes associated with mtDNA-CN may help maintain the levels of mtDNA-CN and preserve mitonuclear compatibility in African admixed cattle. In fact, an increase in mtDNA-CN could trigger a repair response to mitochondrial damage and disruption of mitochondrial function (Nishiyama et al. 2010; Holmbeck et al. 2015; Jiang et al. 2017; Filograna et al. 2021). It has been shown that increasing mtDNA-CN can improve pig blastocyst development rates in vitro (Tsai and St John 2016) and rescue fertility in infertile mice (Jiang et al. 2017). Given that the abundance of mtDNA-CN in whole blood cells derives from the white blood cells and platelets (Picard 2021; Rausser et al. 2021; Gupta et al. 2023) and is the result of a complex hematopoiesis and immunological processes in the bone marrow under selection (Picard 2021). The control of hematopoiesis is regarded as an important trait in African cattle to mitigate the effect of disease challenges, including trypanosomiasis (Noyes et al. 2011; Berthier et al. 2016; Kim et al. 2017, 2020). An increase in mtDNA-CN in whole blood, besides mitigating the effects of mitonuclear incompatibility, may also enhance African zebu supporting their adaptation to disease challenges (Giordano et al. 2014). Thus, mtDNA-CN may serve as a genetic marker in breeding programs for livestock populations that have gone through sex-biased admixture.
Nuclear Compensation Under Extended Mitonuclear DNA Discordance
The observed increase in mitonuclear DNA discordance in African admixed cattle genomes (Figs. 3a and 6), attributed to sex-biased admixture occurring approximately 1,400 years ago (Fig. 2a), provides a model for studying nuclear compensation in relation to mitonuclear incompatibility. Past empirical studies and simulations have focused on changes in genic coding regions and protein functions at or above the species level (Barreto and Burton 2013; Barreto et al. 2018; Princepe and de Aguiar 2024). The origin of African admixed cattle (Fig. 2a) involves parental ancestries with relatively short evolutionary divergence and admixture history (Porto-Neto et al. 2013; Kim et al. 2020; Low et al. 2020). Our results suggest that the regulation of gene expression, influenced by the regulatory elements in parental genomes, contributes to nuclear compensation mechanisms in African admixed cattle (Fig. 6). An evolutionary timescale should be considered when exploring nuclear compensation mechanisms. Our results suggest that the enrichment of zebu ancestry in nuclear genes shaped by selection potentially increased mtDNA-CN and improved mitochondrial function to avoid a cascade of fitness costs due to mitonuclear incompatibility. This scenario has been supported by simulation studies that have shown that under enlarged mitonuclear mismatches, increased selection in incompatible nuclear genes (e.g. from paternal rather than maternal donors) promotes nuclear compensation to preserve mitonuclear compatibility (Princepe and de Aguiar 2024). Given the multiple essential roles of mitochondria in organisms (Mills et al. 2017; Despres 2019; Signorile et al. 2019), it is challenging to distinguish between internal selection (e.g. mitonuclear interactions) (Hill 2019a, 2020) and external adaptation (e.g. environmental stress and human management) (Berthier et al. 2016; Kim et al. 2017, 2020) as drivers of nuclear compensation.
Adaptive Introgression from Ancient African Aurochs B. primigenius africanus
Kwon et al. (2022) proposed 21 selective genes with taurine ancestry to support the hypothesis of mitonuclear coevolution in African cattle (Kwon et al. 2022). We re-checked 21 genes and found that 9 of them presented no significant enrichment in taurine ancestry (Additional file 2: supplementary fig. S5a, Supplementary Material online). However, in the case of CASP8 and TRAK2 (chr2: 87,864,908 to 90,470,906), our findings were in agreement with Kwon et al. (2022) (Additional file 2: supplementary fig. S5, Supplementary Material online). Their evolutionary trajectory suggests they might derive their ancestry from the extinct African aurochs B. p. africanus (Fig. 7). Recent evidence suggests that indigenous African taurine cattle such as Muturu received gene flow from archaic introgression, possibly from African aurochs B. p. africanus (Ginja et al. 2023; Kim et al. 2023). It is possible that this genetic legacy was further introduced to modern African admixed cattle from African taurine cattle following the admixture with Asian zebu. The gene CASP8 is involved in regulating programmed cell death pathways, inflammation, and innate immunity, serving as the initial defense against pathogens (Orning and Lien 2021; Wang et al. 2021). Compared with domestic cattle, the longer history of the African aurochs B. p. africanus on the continent (Verdugo et al. 2019; Kim et al. 2023) could have resulted in local environmental adaptation to various endemic parasites and epidemic diseases in Africa (Decker et al. 2014; Refolo et al. 2020; Mauki et al. 2022; Chen et al. 2023; Kambal et al. 2023; Xia et al. 2023). Thus, CASP8 ancestry in African admixed cattle might be originally associated with infectious disease challenges rather than the alleviation of mitonuclear incompatibilities.
In conclusion, our study provides new insights into the genetic mitonuclear incompatibility in sex-biased admixture and underscores the value of indigenous African admixed cattle as a model for investigating evolutionary genomics and dynamics. Increasing sample sizes could enhance the statistical power of studies on sex-biased admixture and post-admixture drift/selection (Daetwyler et al. 2014; Moran et al. 2021), particularly by including more high-coverage ancient genomes (Brunson et al. 2023; Erven et al. 2024). Further investigations to understand aspects of mitonuclear interplay, such as mitochondrial function (Wolff et al. 2014; Ma et al. 2016; Li et al. 2024), the regulation of mitochondria-related gene expression (Barreto et al. 2015; Ding et al. 2021), and mtDNA-CN variation (Picard 2021), should be conducted in African admixed cattle using more phenotypic and multiomics data (Liu et al. 2022). These efforts will not only deepen our understanding of the evolutionary history and dynamics of African cattle but also help the sustainable development of the species on the continent.
Materials and Methods
Genomic Data
A comprehensive list of the accession numbers of the 258 cattle genomes analyzed in this study is presented in Additional file 1 (supplementary table S1, Supplementary Material online) (PRJNA312138, PRJNA574857, PRJNA386202, PRJNA312139, PRJNA210521, PRJNA210523, PRJNA507259, CNP0000189, PRJNA379859, PRJCA013594, PRJNA422979, PRJNA318087, and PRJNA318089). The genomic data included 17 African cattle breeds (n = 170), most of them classified as African humped zebu but with also one African sanga breed (Ankole), two African zenga breeds (Fogera, Horro), one recent admixed humped zebu × humpless taurine cattle (Sheko), and two African humpless taurine breeds (Muture and N’Dama). Namely, we studied Afar (n = 9); Ankole (n = 10); Arsi (n = 10); Barka (n = 9); Butana (n = 20); Ethiopian Boran (n = 10); Fogera (n = 9); Goffa (n = 10); Horro (n = 11); Kenana (n = 9); Kenyan Boran (n = 10); Mursi (n = 10); Ogaden (n = 9); Sheko (n = 9); White Fulani (n = 5); two African taurine breeds (n = 20): Muturu (n = 10); and N’Dama (n = 10). We also include 12 Indian zebu breeds (n = 30): Nelore (n = 4); Hariana (n = 3); Cholistani (n = 2); Gabrali (n = 2); Achai (n = 2); Bhagnari (n = 3); Dajal (n = 1); Dhanni (n = 2); Lohani (n = 5); Red Sindhi (n = 1); Sahiwal (n = 2); and Tharparkar (n = 3); three European taurine breed (n = 30): Holstein (n = 10); Angus (n = 10); and Jersey (n = 10); one Asian taurine breed (n = 10, Hanwoo); three East Asian zebu breeds (n = 18): Mangshi zebu (n = 10); Wannan zebu (n = 5); and Jinjiang zebu (n = 3).
We obtained qualified clean reads of individual samples (quality of reads > 20) using Trimommatic v.0.38 (Bolger et al. 2014), and mapped them to the current bovine genome reference ARS-UCD1.2 (Rosen et al. 2020) using bwa mem v.0.7.17 (Li and Durbin 2009). The resulting bam files were sorted by samtools v.1.3.1 (Li et al. 2009), and PCR duplicates were removed by Sambamba v.0.8.1 (Tarasov et al. 2015). The “HaplotypeCaller” and “GenotypeGVCFs” tools of GATK (Genome Analysis Toolkit) v.3.8 (McKenna et al. 2010) were used for SNP calling. The detected variants were filtered by the “VariantFiltration” tool with the parameters “QD < 2.0, FS > 60.0, MQ < 40.0, MQRankSum < -12.5, ReadPosRankSum < -8.0”. Subsequently, we retained SNP sites with missing genotype rates <10% and filtered SNPs with minor allele frequencies <0.05 using VCFtools v.0.1.17 (Danecek et al. 2011). Finally, 24.2 million autosomal SNPs were retained for downstream analyses.
Analysis of Population Structure
To identify potential donor populations of African admixed populations, we reconstructed their genetic background using 24.2 million autosomal SNPs from published whole-genome sequencing data for 258 cattle samples (Additional file 1: supplementary table S1, Supplementary Material online). We pruned SNPs with the “-indep-pairwise 50 10 0.2” option following the recommendations of PLINK v.1.9 (Purcell et al. 2007), performed PCA using a smartpca module in EIGENSOFT v.6.14 (Price et al. 2006), estimated individual ancestries at various K values from 2 to 7 using ADMIXTURE v.1.3.0 (Alexander et al. 2009), and constructed an ML tree with FastTree v. 2.1.10 (Price et al. 2009).
Categorization of Genes Involved in Mitonuclear Interactions
To determine the local ancestry deviations of different functional nuclear gene subsets associated with oxidative phosphorylation, we selected from the MitoCarta 3.0 (Rath et al. 2021) database resource, a subset of genes involved in mammalian mitochondrial and nuclear genome interplay. We compiled a list of 1,136 bovine mitonuclear genes, corresponding one-to-one with taurine orthologs of N-mt related genes in humans and mice, and identified four gene subsets involved in mitonuclear interactions: (i) 1,064 mitonuclear genes from MitoCarta 3.0 (Rath et al. 2021); (ii) 149 OXPHOS genes characterized by Signes and Fernandez-Vizarra (2018); (iii) 144 high-mito genes defined by Sloan et al. (2015); and (iv) 21 selective genes reported by Kwon et al. (2022).
Local Ancestry Inference for African Admixed Cattle
We utilized BCFtools v.1.8 (Narasimhan et al. 2016) to generate four SNP datasets corresponding to our four gene subsets. We then calculated FST values of each SNP dataset based on a sliding window approach with windows of 50 kb and a step size of 10 kb using VCFtools v.0.1.17 (Danecek et al. 2011). For Fdm values (degree of local introgression), we included 10 Indian water buffalo (Bubalus bubalus) as an outgroup (Dutta et al. 2020) to estimate local introgression for across the four gene subsets of mitonuclear, high-mito, OXPHOS, selective by Fdm (Manual: https://github.com/simonhmartin/genomics_general). We converted the vcf files to Geno format invoking parseVCF.py command. We adopted ABBABABAwindows.py (Martin et al. 2015) to divide the whole genome into segments of 50 kb windows (10 kb sliding) and estimated Fdm values of each window. Bedtools v.2.29.0 (https://bedtools.readthedocs.io/en/latest/) was used to intersect genome-wide Fdm values of genome-wide with windows spanning 500 bp upstream and downstream of genes within each of the four gene subsets. We obtained four datasets of Fdm values, corresponding to the four gene subsets involved in mitonuclear interactions.
Phasing of SNPs was performed using SHAPEIT v.2.904 (Delaneau et al. 2013). Using Loter (Dias-Alves et al. 2018) and RFmix (Maples et al. 2013), we identified the origin of each haplotype of African admixed cattle as either from African taurine or Asian zebu as 0 or 1, respectively. We then obtained the haplotype values for each gene subset and calculated the mean ratio of taurine ancestry within the regions of each gene subset using a 50 kb window with a 10 kb overlapping slide step. All deviations between each SNP dataset and their genomic background were assessed using the t-test.
Expanded Dataset for Validation of Ancestry
To further validate the ancestry of mitonuclear genes, we used publicly available VCF datasets from 573 African cattle, which included 28 million autosomal SNPs. These datasets encompass 48 African breeds classified as African zebu, African taurine, and admixed populations (https://zenodo.org/records/8317391) (Kambal et al. 2023; Kim et al. 2023). We then merged this VCF dataset with a dataset including 48 Asian zebu (Additional file 1: supplementary table S1, Supplementary Material online) SNP data. We employed VCFtools v.0.1.17 (Danecek et al. 2011) to filter SNPs with missing genotype rates ≥10% and minor allele frequency < 0.05. After removing African cattle breeds with fewer than five individuals, we remained with 36 breeds (Additional file 1: supplementary table S9, Supplementary Material online). Finally, 14.9 million autosomal SNPs and 593 cattle samples were retained for downstream analysis.
To eliminate outliers from potential donor populations, we reconstructed the genetic background of the 593 samples using 14.9 million autosomal SNPs (Additional file 1: supplementary table S9, Supplementary Material online) using ML tree, PCA, and estimated genome-wide ancestries analysis. The ADMIXTURE analysis shows that the Asian zebu genome background dominates the admixed genomes at K = 2 of African admixed (B. taurus × B. indicus) cattle (Additional file 2: supplementary fig. S6, Supplementary Material online). These results are clearly supported by PCA and ML tree analysis (Additional file 2: supplementary fig. S6, Supplementary Material online). Individuals with a foreign genetic ancestry proportion of <5% were retained as potential donor populations for the African admixed populations. Furthermore, we inferred local ancestry deviations in African admixed cattle across the four mtDNA interplay gene subsets by estimating of FST values, Fdm values, Loter ratio, and RFmix ratio.
To examine whether the deviations in local ancestry observed in African admixed cattle are replicated in crossbred cattle populations from other geographic regions, we analyzed VCF datasets (Additional file 1: supplementary table S8, Supplementary Material online) of two admixed Asian taurine cattle breeds: Jiaxian Red cattle (Xia et al. 2021) and Qinchuan cattle (Mei et al. 2019). In these two Asian admixed taurine breeds, some individuals had the characteristics of mismatched mitonuclear genotypes, with zebu ancestry ratios ranging from 29.2% to 41.2%, while their mitochondrial haplotypes were assigned to haplogroup I (Xia et al. 2021; Chen et al. 2023). The ADMIXTURE and PCA analysis show a significant influence of taurine ancestry in the admixed genomes of these two Asian taurine breeds (Additional file 2: supplementary fig. S12a and b, Supplementary Material online). Overall, all Asian admixed taurines shared more than 50% of taurine ancestry at K = 2, with ranging between 72.41% and 81.85% for Qinchuan cattle and ranging between 53.15% and 85.93% for Jiaxian Red cattle (Additional file 2: supplementary fig. S12a, Supplementary Material online). Retrospectively, this data can serve as a reverse control to investigate the universality of the patterns of deviations in local ancestry. We thus reconstructed the genetic background of these two Asian admixed taurine breeds and inferred their deviations in the local ancestry. We then compared the similarities and differences in the local ancestry deviations for the African and Asian cattle datasets.
Mitochondrial DNA Genomic Analysis
The clean mtDNA reads were mapped to the ARS-UCD1.2 (Accession No. NC_006853) (Rosen et al. 2020) mtDNA reference genome. We assemble mtDNA genomes of 258 (African zebu and admixed cattle) and 95 (Asian taurine) cattle using Megahit v1.2.9 (Li et al. 2016). The mtDNA haplogroups were identified with MitoToolPy with referencing DomeTree (http://dometree.kiz.ac.cn) (Peng et al. 2015). Using MAFFT v.7.49 (Yang 2007), we calculated the nonsynonymous/synonymous substitution ratio (ω = dN/dS) for 13 mitochondrial OXPHOS protein genes based on the CODEML branch-site models MA (ω > 1) vs. MA (ω = 1). To estimate the mtDNA-CN, we compared the relative abundance of mtDNA to nuclear DNA (Reznik et al. 2016; Zaidi and Makova 2019), genome coverage (average depth) was calculated using samtools v.1.3.1 (Li et al. 2009). The mtDNA-CN was then calculated using the formula (Ding et al. 2015):
Samples whose genomic DNA was not extracted from whole blood were discarded as outliers. The t-test was used to assess whether the deviations in mtDNA-CN were significant between each population. We calculated correlations between mtDNA-CN and the ratio of taurine ancestry in African admixed cattle with the geom_smooth function of ggplot2 R package (Ito and Murphy 2013).
Genome-Wide Association Study
To investigate whether the level of taurine ancestry is correlated to mtDNA-CN in African admixed cattle, we performed a sequence-based GWAS for mtDNA-CN variations in blood. We analyzed whole-genome sequence data from blood tissues of 116 African admixed breeds, including 11 African zebu breeds (Butana, Kenana, Horro, Arsi, Fogera, Goffa, Ethiopian Boran, Afar, Barka, Kenyan Boran, and Ogaden) with taurine ancestry of <25%. We then calculated the heritability of mtDNA-CN using the TCGA software (Yang et al. 2011). We utilized the values of mtDNA-CN as the quantitative trait for GWAS implementing a univariate linear mixed model with default parameters in the genome-wide efficient mixed-model association method (GEMMA: https://github.com/genetics-statistics/GEMMA) (Zhou and Stephens 2012). The quantile-quantile and Manhattan plots were generated with the ggplot2 R package (Ito and Murphy 2013).
The GEMMA software was used to estimate the phenotypic variance explained by the significant SNPs (Zhou and Stephens 2012). Significant SNPs were analyzed with functional annotations using ANNOVAR software (Yang et al. 2011). Functional enrichment analysis was performed with Metascape (https://metascape.org/) (Zhou et al. 2019) across multiple databases and ontologies to identify the KEGG pathways and gene ontology biological process terms associated with the genes present in the significant candidate regions identified by GWAS. We curated all candidate genes associated with mitochondrial function, metabolism, copy numbers, and assembly and inferred the ancestry deviations of these genes in African admixed cattle. To investigate the involvement of these genes in mtDNA-CN in African admixed cattle, we performed a selective sweep using SweeD (Pavlidis et al. 2013) and considered the top 1% of all likelihood ratio values as indicators of significant selection footprints. Candidate genes under selection and enriched with zebu ancestry were considered to influence variations in mtDNA-CN.
Scanning for Adaptive Introgression
SweeD (Pavlidis et al. 2013) was used to investigate signatures of selection at autosomal regions for mitonuclear genes with taurine ancestry. We considered the top 1% of all likelihood ratio values to determine significant selection sweeps. To further trace the origin of these selective nuclear genes, we used RFmix (Maples et al. 2013) to infer the ancestry of the genes and constructed ML trees based on the genomic fragments under selection using MEGA11 (Tamura et al. 2021). Significant divergence was observed between lineages of African and Eurasian taurine ancestries (e.g. genomic fragment chr2: 87,864,908 to 90,470,906). Several studies suggest that approximately 20% of the genetic ancestry in African taurine might have originated from African aurochs or archaic cattle introgression (Ginja et al. 2023; Kim et al. 2023). To test whether the selective nuclear genes were introgressed with fragments from extinct African aurochs, we utilized ancient DNA data to investigate the origins of the candidate fragments (Verdugo et al. 2019; Kim et al. 2023). We calculated FST between the genomic fragments enriched with African taurine ancestry and ancient DNA genomes from each period (Neolithic Balkans, Anatolian and Iranian cattle represent the neolithic and bronze ages and Levantine cattle represent bronze and iron ages) (Additional file 1: supplementary table S7, Supplementary Material online) based on a sliding window approach with windows of 50 kb and a step size of 10 kb using VCFtools v.0.1.17 (Danecek et al. 2011). We then counted the genotype frequency of each cattle group (Additional file 1: supplementary table S7, Supplementary Material online) using VCFtools v.0.1.17 (Danecek et al. 2011). Haplotype networks and phylogenetic tree were constructed using SNPs that were shared with African aurochs Th7 in the candidate genomic region under selection (chr2: 87,864,908 to 90,470,906).
Supplementary Material
Acknowledgments
M.-S.P. is supported by the Yunnan Revitalization Talent Support Program. The Chinese Government contribution to CAAS-ILRI Joint Laboratory on Livestock and Forage Genetic Resources in Beijing (2023-YWF-ZX-02) and to ICARDA (through J.M.M.) is also appreciated. ILRI and ICARDA wish to acknowledge the generous support of the donors to the CGIAR Trust Fund and the CGIAR Research Program on Livestock. O.H. also acknowledges the support of the University of Nottingham (UK) and, together with J.M.M, the funders of the Centre for Tropical Livestock Genetics and Health (CTLGH).
Contributor Information
Xian Shi, State Key Laboratory of Genetic Evolution and Animal Models and Yunnan Key Laboratory of Molecular Biology of Domestic Animals, Kunming Institute of Zoology, Chinese Academy of Sciences, Kunming, China; Kunming College of Life Science, University of Chinese Academy of Sciences, Kunming, China; Sino-Africa Joint Research Center, Chinese Academy of Sciences, Kunming, China.
Cheng Ma, State Key Laboratory of Genetic Evolution and Animal Models and Yunnan Key Laboratory of Molecular Biology of Domestic Animals, Kunming Institute of Zoology, Chinese Academy of Sciences, Kunming, China; Department of Medical Biochemistry and Microbiology, Uppsala University, Uppsala, Sweden.
Ningbo Chen, Key Laboratory of Animal Genetics Breeding and Reproduction of Shaanxi Province, College of Animal Science and Technology, Northwest A&F University, Yangling, China.
Ming-Min Xu, State Key Laboratory of Genetic Evolution and Animal Models and Yunnan Key Laboratory of Molecular Biology of Domestic Animals, Kunming Institute of Zoology, Chinese Academy of Sciences, Kunming, China; Kunming College of Life Science, University of Chinese Academy of Sciences, Kunming, China.
Sumaya Kambal, Livestock Genetics, International Livestock Research Institute (ILRI), Addis Ababa, Ethiopia; Department of Genetics and Animal Breeding, Faculty of Animal Production, University of Khartoum, Khartoum, Sudan; Department of Bioinformatics and Biostatistics, National University, Khartoum, Sudan.
Zheng-Fei Cai, State Key Laboratory of Genetic Evolution and Animal Models and Yunnan Key Laboratory of Molecular Biology of Domestic Animals, Kunming Institute of Zoology, Chinese Academy of Sciences, Kunming, China; State Key Laboratory for Conservation and Utilization of Bio-Resources in Yunnan, School of Life Sciences, Yunnan University, Kunming, China.
Qiwen Yang, Key Laboratory of Animal Genetics Breeding and Reproduction of Shaanxi Province, College of Animal Science and Technology, Northwest A&F University, Yangling, China.
Adeniyi C Adeola, State Key Laboratory of Genetic Evolution and Animal Models and Yunnan Key Laboratory of Molecular Biology of Domestic Animals, Kunming Institute of Zoology, Chinese Academy of Sciences, Kunming, China; Sino-Africa Joint Research Center, Chinese Academy of Sciences, Kunming, China; Centre for Biotechnology Research, Bayero University, Kano, Nigeria.
Li-Sheng Liu, State Key Laboratory of Genetic Evolution and Animal Models and Yunnan Key Laboratory of Molecular Biology of Domestic Animals, Kunming Institute of Zoology, Chinese Academy of Sciences, Kunming, China; Kunming College of Life Science, University of Chinese Academy of Sciences, Kunming, China.
Jun Wang, Key Laboratory of the Animal Production, Product Quality and Security, Ministry of Education, Jilin Agricultural University, Changchun, Jilin, China; Jilin Provincial International Joint Research Center of Animal Breeding and Reproduction Technology, Jilin Agricultural University, Changchun, Jilin, China; College of Animal Science and Technology, Jilin Agricultural University, Changchun, Jilin, China.
Wen-Fa Lu, Key Laboratory of the Animal Production, Product Quality and Security, Ministry of Education, Jilin Agricultural University, Changchun, Jilin, China; Jilin Provincial International Joint Research Center of Animal Breeding and Reproduction Technology, Jilin Agricultural University, Changchun, Jilin, China; College of Animal Science and Technology, Jilin Agricultural University, Changchun, Jilin, China.
Yan Li, State Key Laboratory for Conservation and Utilization of Bio-Resources in Yunnan, School of Life Sciences, Yunnan University, Kunming, China.
George M Msalya, Department of Animal, Aquaculture, and Range Sciences, Sokoine University of Agriculture, Morogoro, Tanzania.
Chuzhao Lei, Key Laboratory of Animal Genetics Breeding and Reproduction of Shaanxi Province, College of Animal Science and Technology, Northwest A&F University, Yangling, China.
Joram M Mwacharo, Animal and Veterinary Sciences, SRUC and Centre for Tropical Livestock Genetics and Health (CTLGH), Edinburgh, UK; Small Ruminant Genomics, International Centre for Agricultural Research in the Dry Areas (ICARDA), Addis Ababa, Ethiopia.
Jian-Lin Han, Yazhouwan National Laboratory, Sanya, China.
Olivier Hanotte, Livestock Genetics, International Livestock Research Institute (ILRI), Addis Ababa, Ethiopia; Centre for Tropical Livestock Genetics and Health (CTLGH), International Livestock Research Institute (ILRI), Addis Ababa, Ethiopia; School of Life Sciences, University of Nottingham, Nottingham, UK.
Ya-Ping Zhang, State Key Laboratory of Genetic Evolution and Animal Models and Yunnan Key Laboratory of Molecular Biology of Domestic Animals, Kunming Institute of Zoology, Chinese Academy of Sciences, Kunming, China; Kunming College of Life Science, University of Chinese Academy of Sciences, Kunming, China; Sino-Africa Joint Research Center, Chinese Academy of Sciences, Kunming, China; State Key Laboratory for Conservation and Utilization of Bio-Resources in Yunnan, School of Life Sciences, Yunnan University, Kunming, China.
Min-Sheng Peng, State Key Laboratory of Genetic Evolution and Animal Models and Yunnan Key Laboratory of Molecular Biology of Domestic Animals, Kunming Institute of Zoology, Chinese Academy of Sciences, Kunming, China; Kunming College of Life Science, University of Chinese Academy of Sciences, Kunming, China; Sino-Africa Joint Research Center, Chinese Academy of Sciences, Kunming, China.
Supplementary Material
Supplementary material is available at Molecular Biology and Evolution online.
Author Contributions
M.-S.P., Y.-P.Z., O.H., and J.-L.H. designed and conceived the project. X.S., C.M., and M.-M.X. performed comparative population genomic analysis. X.S., Z.-F.C., and M.-M.X. conducted mtDNA analysis. N.C., O.H., Q.Y., L.-S.L., and S.K. expanded data analysis. X.S., M.-S.P., and Z.-F.C. interpreted the results. X.S., M.-S.P., C.M., and M.-M.X. wrote the manuscript. Y.-P.Z., O.H., J.H., N.C., C.L., J.W., W.-F.L., Y.L., J.M.M., G.M.M., and A.C.A. revised the manuscript. All authors contributed and approved the final manuscript.
Funding
This work was supported by the National Key Research and Development Program of China (2023YFF1001000), the National Natural Science Foundation of China (NSFC32388102 and NSFC32341054), and the Sino-Africa Joint Research Center, Chinese Academy of Sciences (SAJC202103).
Data Availability
All data generated or analyzed in this study are included in this publication and in supplementary information. NCBI Sequence Read Archive Biosample IDs of whole-genome sequencing data are available in Additional file 1.
References
- Albrechtsen A, Nielsen FC, Nielsen R. Ascertainment biases in SNP chips affect measures of population divergence. Mol Biol Evol. 2010:27(11):2534–2547. 10.1093/molbev/msq148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander DH, Novembre J, Lange K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 2009:19(9):1655–1664. 10.1101/gr.094052.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey LJ, Doherty AJ. Mitochondrial DNA replication: a PrimPol perspective. Biochem Soc Trans. 2017:45(2):513–529. 10.1042/BST20160162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreto FS, Burton RS. Evidence for compensatory evolution of ribosomal proteins in response to rapid divergence of mitochondrial rRNA. Mol Biol Evol. 2013:30(2):310–314. 10.1093/molbev/mss228. [DOI] [PubMed] [Google Scholar]
- Barreto FS, Pereira RJ, Burton RS. Hybrid dysfunction and physiological compensation in gene expression. Mol Biol Evol. 2015:32(3):613–622. 10.1093/molbev/msu321. [DOI] [PubMed] [Google Scholar]
- Barreto FS, Watson ET, Lima TG, Willett CS, Edmands S, Li W, Burton RS. Genomic signatures of mitonuclear coevolution across populations of Tigriopus californicus. Nat Ecol Evol. 2018:2(8):1250–1257. 10.1038/s41559-018-0588-1. [DOI] [PubMed] [Google Scholar]
- Berthier D, Breniere SF, Bras-Goncalves R, Lemesre JL, Jamonneau V, Solano P, Lejon V, Thevenon S, Bucheton B. Tolerance to trypanosomatids: a threat, or a key for disease elimination? Trends Parasitol. 2016:32(2):157–168. 10.1016/j.pt.2015.11.001. [DOI] [PubMed] [Google Scholar]
- Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014:30(15):2114–2120. 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfiglio S, Ginja C, De Gaetano A, Achilli A, Olivieri A, Colli L, Tesfaye K, Agha SH, Gama LT, Cattonaro F, et al. Origin and spread of Bos taurus: new clues from mitochondrial genomes belonging to haplogroup T1. PLoS One. 2012:7(6):e38601. 10.1371/journal.pone.0038601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet T, Leblois R, Rousset F, Crochet PA. A reassessment of explanations for discordant introgressions of mitochondrial and nuclear genomes. Evolution. 2017:71(9):2140–2158. 10.1111/evo.13296. [DOI] [PubMed] [Google Scholar]
- Brunson K, Witt KE, Monge S, Williams S, Peede D, Odsuren D, Bukhchuluun D, Cameron A, Szpak P, Amartuvshin C, et al. Ancient Mongolian aurochs genomes reveal sustained introgression and management in East Asia. bioRxiv. 10.1101/2023.08.10.552443, 10 August 2023, preprint: not peer reviewed. [DOI]
- Chang TC-T. Functional characterization of bioactive lipids and microproteins. San Diego: University of California; 2022. [Google Scholar]
- Chen N, Cai Y, Chen Q, Li R, Wang K, Huang Y, Hu S, Huang S, Zhang H, Zheng Z, et al. Whole-genome resequencing reveals world-wide ancestry and adaptive introgression events of domesticated cattle in East Asia. Nat Commun. 2018:9(1):2337. 10.1038/s41467-018-04737-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N, Xia X, Hanif Q, Zhang F, Dang R, Huang B, Lyu Y, Luo X, Zhang H, Yan H, et al. Global genetic diversity, introgression, and evolutionary adaptation of indicine cattle revealed by whole genome sequencing. Nat Commun. 2023:14(1):7803. 10.1038/s41467-023-43626-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Lin B-Z, Baig M, Mitra B, Lopes RJ, Santos AM, Magee DA, Azevedo M, Tarroso P, Sasazaki S, et al. Zebu cattle are an exclusive legacy of the South Asia neolithic. Mol Biol Evol. 2010:27(1):1–6. 10.1093/molbev/msp213. [DOI] [PubMed] [Google Scholar]
- Ciesielski GL, Oliveira MT, Kaguni LS. Animal mitochondrial DNA replication. Enzymes. 2016:39:255–292. 10.1016/bs.enz.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutter AD. The polymorphic prelude to Bateson-Dobzhansky-Muller incompatibilities. Trends Ecol Evol. 2012:27(4):209–218. 10.1016/j.tree.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Daetwyler HD, Capitan A, Pausch H, Stothard P, van Binsbergen R, Brondum RF, Liao X, Djari A, Rodriguez SC, Grohs C, et al. Whole-genome sequencing of 234 bulls facilitates mapping of monogenic and complex traits in cattle. Nat Genet. 2014:46(8):858–865. 10.1038/ng.3034. [DOI] [PubMed] [Google Scholar]
- Danecek P, Auton A, Abecasis G, Albers CA, Banks E, DePristo MA, Handsaker RE, Lunter G, Marth GT, Sherry ST, et al. The variant call format and VCFtools. Bioinformatics. 2011:27(15):2156–2158. 10.1093/bioinformatics/btr330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker JE, McKay SD, Rolf MM, Kim J, Molina Alcala A, Sonstegard TS, Hanotte O, Gotherstrom A, Seabury CM, Praharani L, et al. Worldwide patterns of ancestry, divergence, and admixture in domesticated cattle. PLoS Genet. 2014:10(3):e1004254. 10.1371/journal.pgen.1004254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaneau O, Zagury JF, Marchini J. Improved whole-chromosome phasing for disease and population genetic studies. Nat Methods. 2013:10(1):5–6. 10.1038/nmeth.2307. [DOI] [PubMed] [Google Scholar]
- Deremiens L, Schwartz L, Angers A, Glémet H, Angers B. Interactions between nuclear genes and a foreign mitochondrial genome in the redbelly dace Chrosomus eos. Comp Biochem Physiol B Biochem Mol Biol. 2015:189:80–86. 10.1016/j.cbpb.2015.08.002. [DOI] [PubMed] [Google Scholar]
- Despres L. One, two or more species? Mitonuclear discordance and species delimitation. Mol Ecol. 2019:28(17):3845–3847. 10.1111/mec.15211. [DOI] [PubMed] [Google Scholar]
- Dias-Alves T, Mairal J, Blum MGB. Loter: a software package to infer local ancestry for a wide range of species. Mol Biol Evol. 2018:35(9):2318–2326. 10.1093/molbev/msy126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J, Sidore C, Butler TJ, Wing MK, Qian Y, Meirelles O, Busonero F, Tsoi LC, Maschio A, Angius A, et al. Assessing mitochondrial DNA variation and copy number in lymphocytes of ∼2,000 sardinians using tailored sequencing analysis tools. PLoS Genet. 2015:11(7):e1005306. 10.1371/journal.pgen.1005306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Chen W, Li Q, Rossiter SJ, Mao X. Mitonuclear mismatch alters nuclear gene expression in naturally introgressed Rhinolophus bats. Front Zool. 2021:18(1):42. 10.1186/s12983-021-00424-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta P, Talenti A, Young R, Jayaraman S, Callaby R, Jadhav SK, Dhanikachalam V, Manikandan M, Biswa BB, Low WY, et al. Whole genome analysis of water buffalo and global cattle breeds highlights convergent signatures of domestication. Nat Commun. 2020:11(1):4739. 10.1038/s41467-020-18550-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison CK, Burton RS. Cytonuclear conflict in interpopulation hybrids: the role of RNA polymerase in mtDNA transcription and replication. J Evol Biol. 2010:23(3):528–538. 10.1111/j.1420-9101.2009.01917.x. [DOI] [PubMed] [Google Scholar]
- Ellison CK, Burton RS. Interpopulation hybrid breakdown maps to the mitochondrial genome. Evolution. 2008:62(3):631–638. 10.1111/j.1558-5646.2007.00305.x. [DOI] [PubMed] [Google Scholar]
- Erven JAM, Scheu A, Verdugo MP, Cassidy L, Chen N, Gehlen B, Street M, Madsen O, Mullin VE. A high-coverage mesolithic aurochs genome and effective leveraging of ancient cattle genomes using whole genome imputation. Mol Biol Evol. 2024:41(5):msae076. 10.1093/molbev/msae076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filograna R, Mennuni M, Alsina D, Larsson N-G. Mitochondrial DNA copy number in human disease: the more the better? FEBS Lett. 2021:595(8):976–1002. 10.1002/1873-3468.14021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford-Gonzalez D, Hanotte O. Domesticating animals in Africa: implications of genetic and archaeological findings. J World Prehist. 2011:24(1):1–23. 10.1007/s10963-010-9042-2. [DOI] [Google Scholar]
- Ginja C, Guimaraes S, da Fonseca RR, Rasteiro R, Rodriguez-Varela R, Simoes LG, Sarmento C, Belarte MC, Kallala N, Torres JR, et al. Iron age genomic data from Althiburos—Tunisia renew the debate on the origins of African taurine cattle. iScience. 2023:26(7):107196. 10.1016/j.isci.2023.107196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano C, Iommarini L, Giordano L, Maresca A, Pisano A, Valentino ML, Caporali L, Liguori R, Deceglie S, Roberti M, et al. Efficient mitochondrial biogenesis drives incomplete penetrance in Leber's hereditary optic neuropathy. Brain. 2014:137(2):335–353. 10.1093/brain/awt343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R, Kanai M, Durham TJ, Tsuo K, McCoy JG, Kotrys AV, Zhou W, Chinnery PF, Karczewski KJ, Calvo SE, et al. Nuclear genetic control of mtDNA copy number and heteroplasmy in humans. Nature. 2023:620(7975):839–848. 10.1038/s41586-023-06426-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanotte O, Bradley DG, Ochieng JW, Verjee Y, Hill EW, Rege JEO. African pastoralism: genetic imprints of origins and migrations. Science. 2002:296(5566):336–339. 10.1126/science.1069878. [DOI] [PubMed] [Google Scholar]
- Hanotte O, Tawah CL, Bradley DG, Okomo M, Verjee Y, Ochieng J, Rege JE. Geographic distribution and frequency of a taurine Bos taurus and an indicine Bos indicus Y specific allele amongst sub-Saharan African cattle breeds. Mol Ecol. 2000:9(4):387–396. 10.1046/j.1365-294x.2000.00858.x. [DOI] [PubMed] [Google Scholar]
- Havird JC, Forsythe ES, Williams AM, Werren JH, Dowling DK, Sloan DB. Selfish mitonuclear conflict. Curr Biol. 2019:29(11):R496–R511. 10.1016/j.cub.2019.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havird JC, Sloan DB. The roles of mutation, selection, and expression in determining relative rates of evolution in mitochondrial versus nuclear genomes. Mol Biol Evol. 2016:33(12):3042–3053. 10.1093/molbev/msw185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy TM, Burton RS. Strong selective effects of mitochondrial DNA on the nuclear genome. Proc Natl Acad Sci U S A. 2020:117(12):6616–6621. 10.1073/pnas.1910141117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill GE. Mitonuclear coevolution as the genesis of speciation and the mitochondrial DNA barcode gap. Ecol Evol. 2016:6(16):5831–5842. 10.1002/ece3.2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill GE. Mitonuclear ecology. Oxford University Press; 2019a. [Google Scholar]
- Hill GE. Reconciling the mitonuclear compatibility species concept with rampant mitochondrial introgression. Integr Comp Biol. 2019b:59(4):912–924. 10.1093/icb/icz019. [DOI] [PubMed] [Google Scholar]
- Hill GE. Mitonuclear compensatory coevolution. Trends Genet. 2020:36(6):403–414. 10.1016/j.tig.2020.03.002. [DOI] [PubMed] [Google Scholar]
- Holmbeck MA, Donner JR, Villa-Cuesta E, Rand DM. A Drosophila model for mito-nuclear diseases generated by an incompatible interaction between tRNA and tRNA synthetase. Dis Model Mech. 2015:8(8):843–854. 10.1242/dmm.019323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsburgh KA, Prost S, Gosling A, Stanton JA, Rand C, Matisoo-Smith EA. The genetic diversity of the Nguni breed of African cattle (Bos spp.): complete mitochondrial genomes of haplogroup T1. PLoS One. 2013:8(8):e71956. 10.1371/journal.pone.0071956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes AL. Accumulation of slightly deleterious mutations in the mitochondrial genome: a hallmark of animal domestication. Gene. 2013:515(1):28–33. 10.1016/j.gene.2012.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Murphy D. Application of ggplot2 to pharmacometric graphics. CPT Pharmacometrics Syst Pharmacol. 2013:2(10):e79. 10.1038/psp.2013.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Chavarria TE, Yuan B, Lodish HF, Huang NJ. Phosphocholine accumulation and PHOSPHO1 depletion promote adipose tissue thermogenesis. Proc Natl Acad Sci U S A. 2020:117(26):15055–15065. 10.1073/pnas.1916550117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Kauppila TES, Motori E, Li X, Atanassov I, Folz-Donahue K, Bonekamp NA, Albarran-Gutierrez S, Stewart JB, Larsson N-G. Increased total mtDNA copy number cures male infertility despite unaltered mtDNA mutation load. Cell Metab. 2017:26(2):429–436.e4. 10.1016/j.cmet.2017.07.003. [DOI] [PubMed] [Google Scholar]
- Kambal S, Tijjani A, Ibrahim SAE, Ahmed MA, Mwacharo JM, Hanotte O. Candidate signatures of positive selection for environmental adaptation in indigenous African cattle: a review. Anim Genet. 2023:54(6):689–708. 10.1111/age.13353. [DOI] [PubMed] [Google Scholar]
- Kim J, Hanotte O, Mwai OA, Dessie T, Bashir S, Diallo B, Agaba M, Kim K, Kwak W, Sung S, et al. The genome landscape of indigenous African cattle. Genome Biol. 2017:18(1):34. 10.1186/s13059-017-1153-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Kim D, Hanotte O, Lee C, Kim H, Jeong C. Inference of admixture origins in indigenous African cattle. Mol Biol Evol. 2023:40(12):msad257. 10.1093/molbev/msad257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Kwon T, Dessie T, Yoo D, Mwai OA, Jang J, Sung SM, Lee ST, Salim B, Jung JH, et al. The mosaic genome of indigenous African cattle as a unique genetic resource for African pastoralism. Nat Genet. 2020:52(10):1099–1110. 10.1038/s41588-020-0694-2. [DOI] [PubMed] [Google Scholar]
- Kitamata M, Hanawa-Suetsugu K, Maruyama K, Suetsugu S. Membrane-deformation ability of ANKHD1 is involved in the early endosome enlargement. iScience. 2019:17:101–118. 10.1016/j.isci.2019.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch RE, Buchanan KL, Casagrande S, Crino O, Dowling DK, Hill GE, Hood WR, McKenzie M, Mariette MM, Noble DWA, et al. Integrating mitochondrial aerobic metabolism into ecology and evolution. Trends Ecol Evol. 2021:36(4):321–332. 10.1016/j.tree.2020.12.006. [DOI] [PubMed] [Google Scholar]
- Kwon T, Kim K, Caetano-Anolles K, Sung S, Cho S, Jeong C, Hanotte O, Kim H. Mitonuclear incompatibility as a hidden driver behind the genome ancestry of African admixed cattle. BMC Biol. 2022:20(1):20. 10.1186/s12915-021-01206-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane N. Mitonuclear match: optimizing fitness and fertility over generations drives ageing within generations. Bioessays. 2011:33(11):860–869. 10.1002/bies.201100051. [DOI] [PubMed] [Google Scholar]
- Li C-Y, Liu X-C, Li Y-Z, Wang Y, Nie Y-H, Xu Y-T, Zhang X-T, Lu Y, Sun Q. Generation of mitochondrial replacement monkeys by female pronucleus transfer. Zool Res. 2024:45(2):292–298. 10.24272/j.issn.2095-8137.2023.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Luo R, Liu C-M, Leung C-M, Ting H-F, Sadakane K, Yamashita H, Lam T-W. MEGAHIT v1.0: a fast and scalable metagenome assembler driven by advanced methodologies and community practices. Methods. 2016:102:3–11. 10.1016/j.ymeth.2016.02.020. [DOI] [PubMed] [Google Scholar]
- Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009:25(14):1754–1760. 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R; 1000 Genome Project Data Processing Subgroup . The sequence alignment/map format and SAMtools. Bioinformatics. 2009:25(16):2078–2079. 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linseele V, Holdaway SJ, Wendrich W. The earliest phase of introduction of Southwest Asian domesticated animals into Africa. New evidence from the Fayum Oasis in Egypt and its implications. Quat Int. 2016:412:11–21. 10.1016/j.quaint.2015.12.028. [DOI] [Google Scholar]
- Liu S, Gao Y, Canela-Xandri O, Wang S, Yu Y, Cai W, Li B, Xiang R, Chamberlain AJ, Pairo-Castineira E, et al. A multi-tissue atlas of regulatory variants in cattle. Nat Genet. 2022:54(9):1438–1447. 10.1038/s41588-022-01153-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftus RT, MacHugh DE, Bradley DG, Sharp PM, Cunningham P. Evidence for two independent domestications of cattle. Proc Natl Acad Sci U S A. 1994:91(7):2757–2761. 10.1073/pnas.91.7.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low WY, Tearle R, Liu R, Koren S, Rhie A, Bickhart DM, Rosen BD, Kronenberg ZN, Kingan SB, Tseng E, et al. Haplotype-resolved genomes provide insights into structural variation and gene content in Angus and Brahman cattle. Nat Commun. 2020:11(1):2071. 10.1038/s41467-020-15848-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch M. Mutation pressure, drift, and the pace of molecular coevolution. Proc Natl Acad Sci U S A. 2023:120(27):e2306741120. 10.1073/pnas.2306741120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Gutierrez NM, Morey R, Van Dyken C, Kang EJ, Hayama T, Lee Y, Li Y, Tippner-Hedges R, Wolf DP, et al. Incompatibility between nuclear and mitochondrial genomes contributes to an interspecies reproductive barrier. Cell Metab. 2016:24(2):283–294. 10.1016/j.cmet.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacVicar T, Langer T. OPA1 processing in cell death and disease—the long and short of it. J Cell Sci. 2016:129(12):2297–2306. 10.1242/jcs.159186. [DOI] [PubMed] [Google Scholar]
- Maples BK, Gravel S, Kenny EE, Bustamante CD. RFMix: a discriminative modeling approach for rapid and robust local-ancestry inference. Am J Hum Genet. 2013:93(2):278–288. 10.1016/j.ajhg.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SH, Davey JW, Jiggins CD. Evaluating the use of ABBA-BABA statistics to locate introgressed loci. Mol Biol Evol. 2015:32(1):244–257. 10.1093/molbev/msu269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauki DH, Adeola AC, Ng’ang’a SI, Tijjani A, Akanbi IM, Sanke OJ, Abdussamad AM, Olaogun SC, Ibrahim J, Dawuda PM, et al. Genetic variation of Nigerian cattle inferred from maternal and paternal genetic markers. PeerJ. 2021:9:e10607. 10.7717/peerj.10607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauki DH, Tijjani A, Ma C, Ng’ang’a SI, Mark AI, Sanke OJ, Abdussamad AM, Olaogun SC, Ibrahim J, Dawuda PM, et al. Genome-wide investigations reveal the population structure and selection signatures of Nigerian cattle adaptation in the sub-Saharan tropics. BMC Genomics. 2022:23(1):306. 10.1186/s12864-022-08512-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly M, et al. The genome analysis toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010:20(9):1297–1303. 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei C, Wang H, Liao Q, Khan R, Raza SHA, Zhao C, Wang H, Cheng G, Tian W, Li Y, et al. Genome-wide analysis reveals the effects of artificial selection on production and meat quality traits in Qinchuan cattle. Genomics. 2019:111(6):1201–1208. 10.1016/j.ygeno.2018.09.021. [DOI] [PubMed] [Google Scholar]
- Mei C, Wang H, Liao Q, Wang L, Cheng G, Wang H, Zhao C, Zhao S, Song J, Guang X, et al. Genetic architecture and selection of Chinese cattle revealed by whole genome resequencing. Mol Biol Evol. 2018:35(3):688–699. 10.1093/molbev/msx322. [DOI] [PubMed] [Google Scholar]
- Mills EL, Kelly B, O’Neill LAJ. Mitochondria are the powerhouses of immunity. Nat Immunol. 2017:18(5):488–498. 10.1038/ni.3704. [DOI] [PubMed] [Google Scholar]
- Moran BM, Payne C, Langdon Q, Powell DL, Brandvain Y, Schumer M. The genomic consequences of hybridization. eLife. 2021:10:e69016. 10.7554/eLife.69016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran BM, Payne CY, Powell DL, Iverson ENK, Donny AE, Banerjee SM, Langdon QK, Gunn TR, Rodriguez-Soto RA, Madero A, et al. A lethal mitonuclear incompatibility in complex I of natural hybrids. Nature. 2024:626(7997):119–127. 10.1038/s41586-023-06895-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottis A, Herzig S, Auwerx J. Mitocellular communication: shaping health and disease. Science. 2019:366(6467):827–832. 10.1126/science.aax3768. [DOI] [PubMed] [Google Scholar]
- Mwai O, Hanotte O, Kwon Y-J, Cho S. African indigenous cattle: unique genetic resources in a rapidly changing world. Asian-Australas J Anim Sci. 2015:28(7):911–921. 10.5713/ajas.15.0002R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhan V, Danecek P, Scally A, Xue Y, Tyler-Smith C, Durbin R. BCFtools/RoH: a hidden Markov model approach for detecting autozygosity from next-generation sequencing data. Bioinformatics. 2016:32(11):1749–1751. 10.1093/bioinformatics/btw044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama S, Shitara H, Nakada K, Ono T, Sato A, Suzuki H, Ogawa T, Masaki H, Hayashi J, Yonekawa H. Over-expression of Tfam improves the mitochondrial disease phenotypes in a mouse model system. Biochem Biophys Res Commun. 2010:401(1):26–31. 10.1016/j.bbrc.2010.08.143. [DOI] [PubMed] [Google Scholar]
- Noyes H, Brass A, Obara I, Anderson S, Archibald AL, Bradley DG, Fisher P, Freeman A, Gibson J, Gicheru M, et al. Genetic and expression analysis of cattle identifies candidate genes in pathways responding to Trypanosoma congolense infection. Proc Natl Acad Sci U S A. 2011:108(22):9304–9309. 10.1073/pnas.1013486108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orning P, Lien E. Multiple roles of caspase-8 in cell death, inflammation, and innate immunity. J Leukoc Biol. 2021:109(1):121–141. 10.1002/JLB.3MR0420-305R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlidis P, Zivkovic D, Stamatakis A, Alachiotis N. Sweed: likelihood-based detection of selective sweeps in thousands of genomes. Mol Biol Evol. 2013:30(9):2224–2234. 10.1093/molbev/mst112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng M-S, Fan L, Shi N-N, Ning T, Yao Y-G, Murphy RW, Wang W-Z, Zhang Y-P. DomeTree: a canonical toolkit for mitochondrial DNA analyses in domesticated animals. Mol Ecol Resour. 2015:15(5):1238–1242. 10.1111/1755-0998.12386. [DOI] [PubMed] [Google Scholar]
- Pereira RJ, Lima TG, Pierce-Ward NT, Chao L, Burton RS. Recovery from hybrid breakdown reveals a complex genetic architecture of mitonuclear incompatibilities. Mol Ecol. 2021:30(23):6403–6416. 10.1111/mec.15985. [DOI] [PubMed] [Google Scholar]
- Picard M. Blood mitochondrial DNA copy number: what are we counting? Mitochondrion. 2021:60:1–11. 10.1016/j.mito.2021.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccinini G, Iannello M, Puccio G, Plazzi F, Havird JC, Ghiselli F. Mitonuclear coevolution, but not nuclear compensation, drives evolution of OXPHOS complexes in bivalves. Mol Biol Evol. 2021:38(6):2597–2614. 10.1093/molbev/msab054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porto-Neto LR, Sonstegard TS, Liu GE, Bickhart DM, Da Silva MV, Machado MA, Utsunomiya YT, Garcia JF, Gondro C, Van Tassell CP. Genomic divergence of zebu and taurine cattle identified through high-density SNP genotyping. BMC Genomics. 2013:14(1):876. 10.1186/1471-2164-14-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prashar A, Bussi C, Fearns A, Capurro MI, Gao X, Sesaki H, Gutierrez MG, Jones NL. Lysosomes drive the piecemeal removal of mitochondrial inner membrane. Nature. 2024:632(8027):1110–1117. 10.1038/s41586-024-07835-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006:38(8):904–909. 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- Price MN, Dehal PS, Arkin AP. FastTree: computing large minimum evolution trees with profiles instead of a distance matrix. Mol Biol Evol. 2009:26(7):1641–1650. 10.1093/molbev/msp077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Princepe D, de Aguiar MAM. Nuclear compensatory evolution driven by mito-nuclear incompatibilities. Proc Natl Acad Sci U S A. 2024:121(42):e2411672121. 10.1073/pnas.2411672121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, Bender D, Maller J, Sklar P, de Bakker PIW, Daly MJ, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007:81(3):559–575. 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiros PM, Mottis A, Auwerx J. Mitonuclear communication in homeostasis and stress. Nat Rev Mol Cell Biol. 2016:17(4):213–226. 10.1038/nrm.2016.23. [DOI] [PubMed] [Google Scholar]
- Rand DM, Haney RA, Fry AJ. Cytonuclear coevolution: the genomics of cooperation. Trends Ecol Evol. 2004:19(12):645–653. 10.1016/j.tree.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Rath S, Sharma R, Gupta R, Ast T, Chan C, Durham TJ, Goodman RP, Grabarek Z, Haas ME, Hung WHW, et al. MitoCarta3.0: an updated mitochondrial proteome now with sub-organelle localization and pathway annotations. Nucleic Acids Res. 2021:49(D1):D1541–D1547. 10.1093/nar/gkaa1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausser S, Trumpff C, McGill MA, Junker A, Wang W, Ho SH, Mitchell A, Karan KR, Monk C, Segerstrom SC, et al. Mitochondrial phenotypes in purified human immune cell subtypes and cell mixtures. eLife. 2021:10:e70899. 10.7554/eLife.70899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refolo G, Vescovo T, Piacentini M, Fimia GM, Ciccosanti F. Mitochondrial interactome: a focus on antiviral signaling pathways. Front Cell Dev Biol. 2020:8:8. 10.3389/fcell.2020.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reist-Marti S, Simianer H, Gibson J, Hanotte O, Rege J. Weitzman’s approach and conservation of breed diversity: an application to African cattle breeds. Conserv Biol. 2003:17(5):1299–1311. 10.1046/j.1523-1739.2003.01587.x. [DOI] [Google Scholar]
- Reznik E, Miller ML, Senbabaoglu Y, Riaz N, Sarungbam J, Tickoo SK, Al-Ahmadie HA, Lee W, Seshan VE, Hakimi AA, et al. Mitochondrial DNA copy number variation across human cancers. eLife. 2016:5:e10769. 10.7554/eLife.10769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha AL, Pai V, Perkins G, Chang T, Ma J, Souza D, Chu EV, Vaughan Q, Diedrich JM, Ellisman JK, et al. An inner mitochondrial membrane microprotein from the SLC35A4 upstream ORF regulates cellular metabolism. J Mol Biol. 2024:436(10):168559. 10.1016/j.jmb.2024.168559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong Z, Tu P, Xu P, Sun Y, Yu F, Tu N, Guo L, Yang Y. The mitochondrial response to DNA damage. Front Cell Dev Biol. 2021:9:669379. 10.3389/fcell.2021.669379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen BD, Bickhart DM, Schnabel RD, Koren S, Elsik CG, Tseng E, Rowan TN, Low WY, Zimin A, Couldrey C, et al. De novo assembly of the cattle reference genome with single-molecule sequencing. Gigascience. 2020:9(3):giaa021. 10.1093/gigascience/giaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumer M, Xu CL, Powell DL, Durvasula A, Skov L, Holland C, Blazier JC, Sankararaman S, Andolfatto P, Rosenthal GG, et al. Natural selection interacts with recombination to shape the evolution of hybrid genomes. Science. 2018:360(6389):656–660. 10.1126/science.aar3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signes A, Fernandez-Vizarra E. Assembly of mammalian oxidative phosphorylation complexes I–V and supercomplexes. Essays Biochem. 2018:62(3):255–270. 10.1042/EBC20170098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signorile A, Sgaramella G, Bellomo F, De Rasmo D. Prohibitins: a critical role in mitochondrial functions and implication in diseases. Cells. 2019:8(1):71. 10.3390/cells8010071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan DB, Fields PD, Havird JC. Mitonuclear linkage disequilibrium in human populations. Proc Biol Sci. 2015:282(1815):20151704. 10.1098/rspb.2015.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan DB, Havird JC, Sharbrough J. The on-again, off-again relationship between mitochondrial genomes and species boundaries. Mol Ecol. 2017:26(8):2212–2236. 10.1111/mec.13959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan DB, Warren JM, Williams AM, Wu Z, Abdel-Ghany SE, Chicco AJ, Havird JC. Cytonuclear integration and co-evolution. Nat Rev Genet. 2018:19(10):635–648. 10.1038/s41576-018-0035-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabara LC, Burr SP, Frison M, Chowdhury SR, Paupe V, Nie Y, Johnson M, Villar-Azpillaga J, Viegas F, Segawa M, et al. MTFP1 controls mitochondrial fusion to regulate inner membrane quality control and maintain mtDNA levels. Cell. 2024:187(14):3619–3637.e27. 10.1016/j.cell.2024.05.017. [DOI] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Kumar S. MEGA11: molecular evolutionary genetics analysis version 11. Mol Biol Evol. 2021:38(7):3022–3027. 10.1093/molbev/msab120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao M, Chen J, Cui C, Xu Y, Xu J, Shi Z, Yun J, Zhang J, Ou G-Z, Liu C, et al. Identification of a longevity gene through evolutionary rate covariation of insect mito-nuclear genomes. Nat Aging. 2024:4(8):1076–1088. 10.1038/s43587-024-00641-z. [DOI] [PubMed] [Google Scholar]
- Tarasov A, Vilella AJ, Cuppen E, Nijman IJ, Prins P. Sambamba: fast processing of NGS alignment formats. Bioinformatics. 2015:31(12):2032–2034. 10.1093/bioinformatics/btv098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toews DP, Brelsford A. The biogeography of mitochondrial and nuclear discordance in animals. Mol Ecol. 2012:21(16):3907–3930. 10.1111/j.1365-294X.2012.05664.x. [DOI] [PubMed] [Google Scholar]
- Tsai T, St John JC. The role of mitochondrial DNA copy number, variants, and haplotypes in farm animal developmental outcome. Domest Anim Endocrin. 2016:56:S133–S146. 10.1016/j.domaniend.2016.03.005. [DOI] [PubMed] [Google Scholar]
- Utsunomiya YT, Milanesi M, Fortes MRS, Porto-Neto LR, Utsunomiya ATH, Silva M, Garcia JF, Ajmone-Marsan P. Genomic clues of the evolutionary history of Bos indicus cattle. Anim Genet. 2019:50(6):557–568. 10.1111/age.12836. [DOI] [PubMed] [Google Scholar]
- Verdugo MP, Mullin VE, Scheu A, Mattiangeli V, Daly KG, Maisano Delser P, Hare AJ, Burger J, Collins MJ, Kehati R, et al. Ancient cattle genomics, origins, and rapid turnover in the Fertile Crescent. Science. 2019:365(6449):173–176. 10.1126/science.aav1002. [DOI] [PubMed] [Google Scholar]
- Wang Y, Karki R, Zheng M, Kancharana B, Lee S, Kesavardhana S, Hansen BS, Pruett-Miller SM, Kanneganti T-D. Cutting edge: caspase-8 is a linchpin in caspase-3 and gasdermin D activation to control cell death, cytokine release, and host defense during influenza A virus infection. J Immunol. 2021:207(10):2411–2416. 10.4049/jimmunol.2100757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JA, McHugo GP, Dover MJ, Hall TJ, Ng’ang’a SI, Sonstegard TS, Bradley DG, Frantz LAF, Salter-Townshend M, MacHugh DE. Genome-wide local ancestry and evidence for mitonuclear coadaptation in African hybrid cattle populations. iScience. 2022:25(7):104672. 10.1016/j.isci.2022.104672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver RJ, Rabinowitz S, Thueson K, Havird JC. Genomic signatures of mitonuclear coevolution in mammals. Mol Biol Evol. 2022:39(11):msac233. 10.1093/molbev/msac233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff JN, Ladoukakis ED, Enriquez JA, Dowling DK. Mitonuclear interactions: evolutionary consequences over multiple biological scales. Philos Trans R Soc Lond B Biol Sci. 2014:369(1646):20130443. 10.1098/rstb.2013.0443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X, Qu K, Wang Y, Sinding MS, Wang F, Hanif Q, Ahmed Z, Lenstra JA, Han J, Lei C, et al. Global dispersal and adaptive evolution of domestic cattle: a genomic perspective. Stress Biol. 2023:3(1):8. 10.1007/s44154-023-00085-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X, Zhang S, Zhang H, Zhang Z, Chen N, Li Z, Sun H, Liu X, Lyu S, Wang X, et al. Assessing genomic diversity and signatures of selection in Jiaxian Red cattle using whole-genome sequencing data. BMC Genomics. 2021:22(1):43. 10.1186/s12864-020-07340-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P, Wang L, Peng H, Liu H, Liu H, Yuan Q, Lin Y, Xu J, Pang X, Wu H, et al. Disruption of HARAS2 in cochlear hair cells causes progressive mitochondrial dysfunction and hearing loss in mice. Front Cell Neurosci. 2021:15:804345. 10.3389/fncel.2021.804345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Lee SH, Goddard ME, Visscher PM. GCTA: a tool for genome-wide complex trait analysis. Am J Hum Genet. 2011:88(1):76–82. 10.1016/j.ajhg.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z. PAML 4: phylogenetic analysis by maximum likelihood. Mol Biol Evol. 2007:24(8):1586–1591. 10.1093/molbev/msm088. [DOI] [PubMed] [Google Scholar]
- Zaidi AA, Makova KD. Investigating mitonuclear interactions in human admixed populations. Nat Ecol Evol. 2019:3(2):213–222. 10.1038/s41559-018-0766-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang QS, Maass DL, Wigginton JG, Barber RC, Martinez B, Idris AH, Horton JW, Nwariaku FE. Burn serum causes a CD14-dependent mitochondrial damage in primary cardiomyocytes. Am J Physiol-Heart C. 2010:298(6):H1951–H1958. 10.1152/ajpheart.00927.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Stephens M. Genome-wide efficient mixed-model analysis for association studies. Nat Genet. 2012:44(7):821–824. 10.1038/ng.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Zhou B, Pache L, Chang M, Khodabakhshi AH, Tanaseichuk O, Benner C, Chanda SK. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun. 2019:10(1):1523. 10.1038/s41467-019-09234-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed in this study are included in this publication and in supplementary information. NCBI Sequence Read Archive Biosample IDs of whole-genome sequencing data are available in Additional file 1.