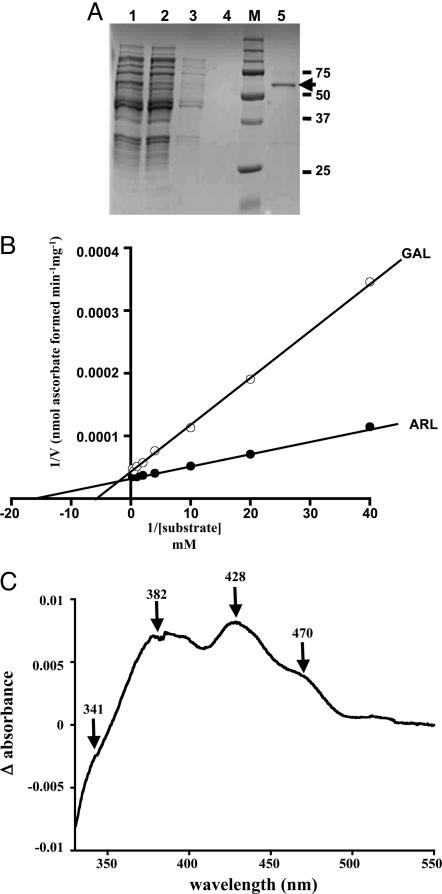
Fig. 3.
Biochemical properties of TbALO. (A) Fractions obtained during the TbALO purification were resolved by SDS/PAGE (10% gel) and visualized after Coomassie staining. A clarified fraction of an E. coli (pTrcHis-TbALO) lysate after 6-h isopropyl β-d-thiogalactoside induction (lane 1) was loaded on a Ni-NTA column and the flow-through collected (lane 2). The column was washed extensively with 20 mM imidazole (lanes 3 and 4) and the recombinant protein eluted with 200 mM imidazole (lane 5). Markers (M) are in kilodaltons. The band of 60 kDa (indicated) corresponds to recombinant TbALO. (B) TbALO activity was assayed by following the ascorbate-dependent reduction of ferricytochrome c (18) in the presence of 1.2 μM TbALO and d-arabinono-γ-lactone (ARL) (0.025–4 mM) or l-galactono-γ-lactone (GAL) (0.025–4 mM) (Materials and Methods). (C) The absorbance spectrum (330–550 nm) of 1 μM TbALO was determined (oxidized). The sample was then saturated with argon and d-arabinono-γ-lactone (2 mM) added. After 2 h, the absorbance spectrum of TbALO (reduced) was examined. The difference between the oxidized and reduced spectra was then plotted.