Abstract
Background
Previous evidence suggests that methionine (Met) consumption can promote placental angiogenesis and improve fetal survival. To investigate the mechanisms by which increased levels of Met as hydroxyl-Met (OHMet) improve placental function, forty sows were divided into four groups and fed either a control diet, or diets supplemented with 0.15% OHMet, 0.3% OHMet or 0.3% Met (n = 10). Placentas were collected immediately after expulsion, and extracted proteins were analyzed by tandem mass tag based quantitative proteomic analysis.
Results
We found that 0.15% OHMet consumption significantly increased placental vascular density compared with the control. Proteomic analysis identified 5,136 proteins, 87 of these were differentially expressed (P < 0.05, |fold change| > 1.2). Enriched pathways in the Kyoto Encyclopedia of Genes and Genomes for 0.15% OHMet vs. control and 0.15% OHMet vs. 0.3% OHMet were glutathione metabolism; for 0.15% OHMet vs. 0.3% Met, they were NOD-like receptor signaling and apoptosis. Further analysis revealed that 0.15% OHMet supplementation upregulated the protein expression of glutathione-S-transferase (GSTT1) in placentas and trophoblast cells compared with the control and 0.3% OHMet groups, upregulated thioredoxin (TXN) in placentas and trophoblast cells compared with the 0.3% OHMet and 0.3% Met groups, and decreased reactive oxygen species (ROS) levels in trophoblast cells compared with other groups. In contrast, sows fed 0.3% OHMet or 0.3% Met diets increased placental interleukin 1β levels compared with the control, and upregulated the protein expression of complex I-B9 (NDUFA3) compared with the 0.15% OHMet group. Furthermore, homocysteine, an intermediate in the trans-sulphuration pathway of Met, damaged placental function by inhibiting the protein expression of TXN, leading to apoptosis and ROS production.
Conclusion
Although dietary 0.15% OHMet supplementation improved placental angiogenesis and increased antioxidative capacity, 0.3% OHMet or 0.3% Met supplementation impaired placental function by aggravating inflammation and oxidative stress, which is associated with cumulative homocysteine levels.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40104-025-01159-z.
Keywords: Angiogenesis, Gestation sow, Hydroxy-methionine analogue, Placenta, TMT Proteomics
Introduction
The placenta plays a crucial role in nourishing the fetus and promoting its growth [1, 2]. Placental activities directly affect the well-being of the fetus [3]. Accumulating evidence indicates that the physiological mechanisms governing placental formation, growth, and development are intricately linked to specific factors such as angiogenesis and antioxidant abilities [4]. These factors significantly influence pregnancy outcomes. Amino acids play a vital role in promoting placental growth by facilitating protein synthesis and supporting trophoblast and endothelial cell proliferation [5]. Growing evidence suggests that methionine (Met), an essential amino acid, improve placental angiogenesis and antioxidant defense [6]. Previous studies showed that the Met intake recommended by the National Research Council (2012) [7] for gestating sows was insufficient for optimal reproductive performance [8, 9]. However, our previous study and others found a progressive increase in maternal homocysteine (Hcy) levels during late gestation [10, 11]. Moreover, elevated dietary Met concentration could lead to Hcy accumulation, thereby disrupting the placental function [12]. Therefore, preventing Hcy accumulation while ensuring Met supply is essential for placental angiogenesis and other functions.
DL-2-hydroxy-4-methylthiobutanoic acid (OHMet), a new source of Met, is widely used in commercial animal feeds [13–15]. Notably, dietary OHMet supplementation accumulates lower Hcy levels when compared to crystalline D-Met, reflecting the metabolic difference between the two sources of Met [13, 16]. Moreover, Met is utilized more in the intestine than OHMet, so Met has a higher first-pass metabolic rate [16]. Our recent studies also demonstrated that dietary 1.5 g/kg OHMet supplementation improved maternal metabolism and increased the fetal survival rate [17]. However, the mechanisms through which different Met sources and levels influence placental function are not fully understood.
Therefore, this study aimed to explore the potential for enhancing placental morphology and function through increased maternal OHMet intake. Placental analysis in sows was performed using tandem mass tag (TMT) based quantitative proteomics, along with assessments of antioxidant capacity, morphology, and gene expression associated with antioxidant and angiogenic functions in porcine trophoblast (pTr) cells. These findings may enhance our understanding of the biological mechanisms that underlie the placental function of maternal Met nutrition.
Materials and methods
The experimental scheme was approved by the Institutional Animal Care and Use Committee of Sichuan Agricultural University (Ya’an, China) (approval No. SYXK-Chuan-2014-184). OHMet was provided by Adisseo (88%, Rhodimet AT88, Adisseo Life Science, Shanghai, China) and the L-Met (99%) was obtained from Cheil Jedang Biochemical Co., Ltd. (Qingdao, China).
Animals, experimental design and diets
Forty primiparous sows [Duroc × (Landrace × Yorkshire)] with similar BW (154.46 ± 1.60 kg, P = 0.85) were selected and randomly assigned into 4 treatments, each with 10 replicates, starting on d 1 of gestation (G1). The average litter size of the sows was 12.13 ± 0.50. All sows were fed an equal amount control (CON) diet until G59. The CON diet was formulated to meet the nutrition requirements of gestating sows, as recommended by the NRC (2012) [7]. The present study was divided into 2 phases: the first phase spanned from G60 to G90, and the second phase from G90 to G114. Sows in the CON group were fed a basal diet containing 3.4 g of Met per kg of feed during phase 1 and 4.5 g during phase 2 (Table 1). The other groups were: the 1.5S-OHMet group, supplemented with 1.5 g OHMet (88% Purity) per kg of feed; the 3.0S-OHMet group, supplemented with 3.0 g OHMet per kg of feed; and the 3.0S-Met group, supplemented with 3.0 g L-Met (99% Purity) per kg of feed.
Table 1.
Ingredients and nutrient composition of the experimental diets (as-fed basis)
| Feedstuff, g/kg | Phase 1 | Phase 2 | Nutrient levela | Phase 1 | Phase 2 |
|---|---|---|---|---|---|
| G60–G90 | G91–G114 | G60–G90 | G91–G114 | ||
| Corn | 689.11 | 642.58 | Gross energy, MJ/kg | 16.51 | 16.52 |
| Wheat bran | 70.00 | 55.00 | Crude protein, % | 10.13 | 13.43 |
| Rice bran meal | 60.00 | 60.00 | SID-Lys, % | 0.52 | 0.69 |
| Soybean meal | 41.50 | 97.00 | SID-Met, % | 0.15 | 0.20 |
| Bean curd | 69.30 | 50.90 | SID Met + Cys, % | 0.34 | 0.45 |
| Fish meal | 20.00 | SID-Thr, % | 0.37 | 0.48 | |
| Soybean oil | 40.00 | 40.00 | SID-Trp, % | 0.09 | 0.13 |
| L-Lys-HCl (98.5%) | 2.602 | 2.324 | SID-Ile, % | 0.30 | 0.36 |
| L-Met (99%) | 0.112 | 0.469 | SID-Val, % | 0.37 | 0.49 |
| L-Thr (98.5%) | 0.892 | 0.966 | Calcium, % | 0.61 | 0.83 |
| Trp | 0.084 | 0.15 | Total phosphorus, % | 0.49 | 0.62 |
| Dicalcium phosphate | 8.041 | 10.528 | STTD-P, % | 0.27 | 0.36 |
| Limestone | 8.733 | 10.461 | |||
| Sodium chloride | 4.00 | 4.00 | |||
| Choline chloride (50%) | 1.70 | 1.70 | |||
| Premixb | 3.925 | 3.925 | |||
| Total | 1,000 | 1,000 |
Gx = day x of gestation
aGross energy is analyzed value and the others are calculated values
bVitamin and mineral mixture for gestation sows supplied the following amounts of vitamins per kg and minerals per kg of complete diet: 6,000 IU vitamin A; 1,200 IU vitamin D3; 50 IU vitamin E; 1 mg vitamin B1; 3.6 mg vitamin B2; 1.8 mg vitamin B6; 12.5 μg vitamin B12; 20 mg niacin; 2 mg folate; 316 mg iron; 183 mg copper; 289 mg zinc; 169 mg manganese; 0.3 mg iodine
Feeding management
Semen was obtained from three adult male pigs (Yorkshire) with of the same batch and age. The vitality of sperm was at least 85%, and the abnormality rate of the sperm did not exceed 10%. Sows were individually housed in pens measuring 2.6 m × 0.6 m from G1 to G107, and then moved to adjustable 2.2 m × 1.5 m farrowing cages. Each sow was housed in a single barn with controlled environmental conditions. Temperatures was maintained between 20–24 °C and humidity levels between 40%–60%. At parturition, the total number of litters, liveborn, mummies, and weak piglets were recorded, and piglet birth weight was measured after farrowing. Two sows were removed from the dataset due to death during farrowing.
Data collection and sampling
Six to eight sows per group were randomly selected for sample collection. Fasting blood samples were collected from sows through the ear vein on the farrowing day. Blood samples were centrifuged at 3,000 × g for 15 min, after which the serum was then separated and stored at −20 °C until analysis, or at −80 °C for cell culture purposes. During delivery, the umbilical cord of each piglet was tied with labeled cotton thread to facilitate placenta matching. Placental samples were immediately collected from piglets whose BW was closest to the average, using the method outlined in previous studies [10, 11]. After the placenta was expelled, two sections of placental tissue were collected, each about 5 cm from the cord insertion point. One section was immediately frozen in liquid nitrogen, and the other was preserved in 4% paraformaldehyde.
Determination of placental vascular density
Tissue samples were initially fixed with a 4% paraformaldehyde solution, embedded in paraffin, and then cut into 5 µm sections. The sections were subsequently stained with hematoxylin and eosin (H&E). Images of three to four fields on each slide with the stained tissues were captured using a Leica DM1000 projection microscope. The placental tissue area was delineated, and the placental vessels within this area were also delineated for the quantification of their area and number. Blood vessels within the stroma were imaged using a 4 × objective and 10 × eyepiece, while other vascular indicators were captured with a 40 × objective and 10 × eyepiece. Image analysis techniques (Image Pro Plus, Media Cybernetic, USA) were used to calculate the relative area of the placental stroma vessels. The width of the placental fold is the average distance from one fold to another. The length of the placental fold is the average distance from the top of all folds to the bottom [18]. The placental vascular density was defined as the area of the blood vessels divided by the area of the stroma, then multiplied by 100%.
Measurement of antioxidative capacity and inflammatory cytokine in placenta
Placental tissue samples (approximately 0.1 g) were homogenized in an ice-cold 0.9% physiological saline solution (1:9, w/v), then centrifuged at 3,500 × g for 15 min at 4 °C. Placental samples were analyzed for glutathione peroxidase (GSH-Px), total superoxide dismutase (T-SOD), malondialdehyde (MDA), catalase (CAT) and induced nitric oxide synthase (iNOS) concentrations, following the method provided by Nanjing Jiancheng (Nanjing, China). Reduced (GSH) and oxidized (GSSG) glutathione were measured using a commercially available kit from Beyotime (Jiangsu, China). Placental tissue concentrations of tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and interleukin-1β (IL-1β) were determined using commercial ELISA kits from Meimian (Jiangsu, China). The intra-assay coefficient of variation was less than 10%, and the inter-assay coefficient was less than 12%. Results were normalized against total protein concentration for comparisons between samples [19].
S-adenosyl-methionine (SAM), S-adenosyl-homocysteine (SAH), and amino acid analysis
The SAM and SAH levels in serum samples were determined using ultra-performance liquid chromatography (UPLC, Waters, USA) equipped with a Waters ACQUITY UPLC BEHC18 column (150 mm × 2.1 mm, 1.7 μm). In brief, 400 μL of the serum sample and 40 μL trichloroacetic acid solution (400 g/L) were mixed and centrifuged at 14,000 × g for 20 min at 4 °C, and the supernatant was collected. The SAM (#A4377) and SAH (#A9384) standards were purchased from Sigma-Aldrich (Missouri, USA). Concurrently, serum amino acid concentrations were determined using an automatic amino acid analyzer (Hitachi L-8800, Hitachi High-Technologies Corporation, Tokyo, Japan) [17].
Placental quantitative proteomics analysis
Samples from each group in the placenta, totaling three per group, were analyzed using the Tandem Mass Tags (TMT) method [20]. Total proteins were extracted from the samples. Following quality assessment (Fig. S1), the samples were labeled with TMT tags, desalted, and lyophilized. Fractions were separated, and liquid chromatography-tandem mass spectrometry (LC–MS/MS) was used to analyze the resulting peptides. After quality control, the protein data were searched against the Sus scrofa protein database using Proteome Discoverer 2.2 (Thermo Fisher Scientific). Protein expression levels were normalized relative to placental samples. The threshold of a fold change ≥ 1.20 (or ≤ 0.83) and a P-value ≤ 0.05 was used to identify differentially expressed proteins (DEPs). DEPs were identified across the four treatment groups. Functional enrichment analysis of the DEPs was conducted using the Gene Ontology (GO) database and the Kyoto Encyclopedia of Genes and Genomes (KEGG) database.
In vitro cell model
The porcine trophoblast cell line (pTr) used in this experiment was previously established and characterized using porcine blastocysts collected on G12 [1]. The pTr cells were cultured in DMEM/F12 (Gibco, Missouri, USA) supplemented with 10% fetal bovine serum (FBS) (Gibco, Missouri, USA) and 1% penicillin–streptomycin liquid (Gibco, Missouri, USA). All maternal serum was filtered using a 0.22-μm sterile filter (Millipore, USA) before cell processing. For maternal serum treatment: pTr cells were seeded in 12-well plates and, after 24 h, the medium was switched from DMEM/F12 with 10% FBS to Met-free DMEM/F12 (Cellverse, Shanghai, China) supplemented with 20% maternal serum from each of the four groups [21]. For Hcy treatment, pTr cells were seeded in 12-well plates. After 24 h, the medium was changed from DMEM/F12 with 10% FBS to DMEM/F12 with varying Hcy concentrations. The cells were collected after 24 h of treatment for further analysis.
Cell viability, proliferation and lactate dehydrogenase release assay
Cell viability was assessed using a CCK-8 kit (Beyotime) according to the manufacturer's protocols. Cell proliferation was determined by using a EdU-555 kit (Beyotime, #C0075S). Apoptosis-induced destruction of the cell membrane structure can result in the release of lactate dehydrogenase (LDH) from the cytoplasm. LDH activity was measured using an assay kit (Beyotime). Fluorescence was measured at 450 nm with a microplate reader. All procedures followed the manufacturer’s instructions.
Intracellular ROS analysis and scratch healing assay
Intracellular levels of reactive oxygen species (ROS) were measured using DCFH-FA (Solarbio, Beijing, China) according to the manufacturer's protocols. pTr cells were seeded in a 6-well plate and cultured to form a confluent monolayer overnight. A scratch wound was then created with a 200-μL pipette tip, and the effect of Hcy on wound healing was measured 24 h post-scratch.
Flow cytometry analysis of apoptosis
The fluorescence intensity of FITC Annexin V and propidium iodide (PI) was analyzed using a flow cytometer (BD, FACSVerse, USA). Apoptosis data were analyzed using FlowJo X software.
Real-time quantitative PCR
Real-time quantitative PCR (RT-qPCR) was performed as follows. Total RNA was extracted from frozen placental samples using Trizol reagent (Sigma-Aldrich). Subsequently, cDNA was synthesized from 1 mg of total RNA using a PrimeScript reverse transcription kit (Takara, Dalian, China), following the manufacturer’s instructions. The cycling conditions were set as 95 °C for 15 s, followed by 40 cycles of 95 °C for 5 s, and then 60 °C for 34 s. Real-time PCR was conducted using a 7900HT ABI Prism system (Applied Biosystems, Foster City, CA, USA) and SYBR Green RT-qPCR reagent (Takara). β-Actin and GAPDH were used as internal reference genes. The RT-qPCR data were analyzed using the 2−ΔΔCt method [22]. Gene expression in the placenta was assessed using an RNase protection assay. Primer sequences for the genes are listed in Table S1.
Western blot analysis
Placental tissues and pTr cells were lysed using cold lysis buffer (Beyotime), centrifuged at 12,000 × g for 30 min at 4 °C, and the supernatant was combined with loading buffer (Bio-Rad, Hercules, USA) and mercaptoethanol, then boiled for 5 min. Samples were separated by SDS-PAGE using 10% or 15% acrylamide gels and transferred onto PVDF membranes. Primary antibodies were thioredoxin (TXN, Zen BioScience, 1:1,000), HADHA (Zen BioScience, 1:1,000), NDUFA3 (Zen BioScience, 1:1,000), ENO3 (Zen BioScience, 1:1,000), GSTT1 (Proteintech, 1:1,000), GAPDH (Absin, 1:1,000), β-actin (Cell Signaling Technology, 1:1,000). The secondary rabbit antibodies (1:2,000) were purchased from Beyotime. The protein bands were quantified by Image Lab (Bio-Rad).
Statistical analysis
The data were analyzed using the PROC MIXED procedure of SAS 9.4 (SAS Inst. Inc., Cary, NC, USA). The Shapiro–Wilk test was conducted to assess the normality of the data. Multiple comparison of data among the groups were performed using Turkey’s tests when the data were normally distributed. Data points identified as outliers by the Grubbs test (α < 0.05) were excluded from the analysis. Proteomic data was analyzed using paired t-test. Pearson’s correlation analysis was used to identify relationships between differential proteins and placental traits, as well as between differential proteins and Met metabolites. Data are presented as means ± standard error of the mean (SEM). Statistical significance was defined as P < 0.05, while a tendency towards difference significance was indicated at 0.05 ≤ P < 0.10.
Results
Effects of increased consumption of Met as OHMet on placental angiogenesis
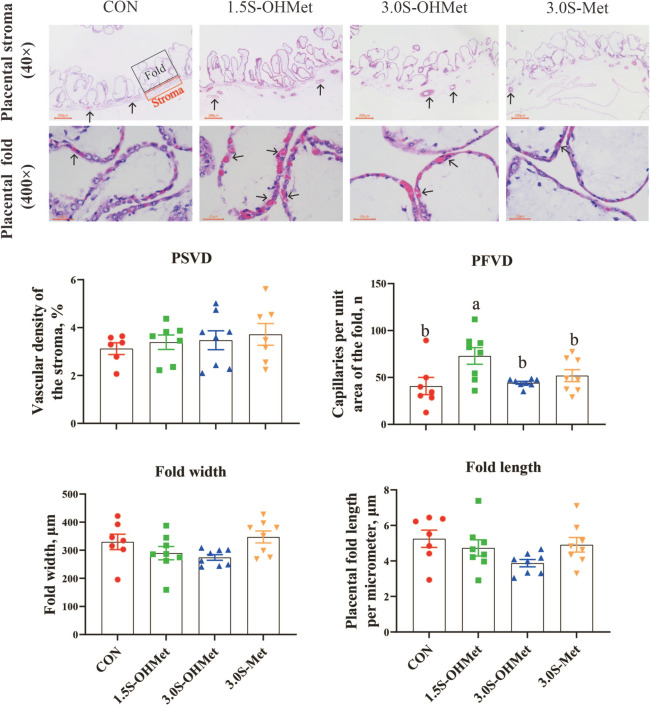
To determine if increased maternal OHMet intake enhanced placental angiogenesis, placental vascular density was assessed. The findings indicated that dietary intake of 1.5S-OHMet elevated the number of capillaries per unit area in the placental fold compared to the CON, 3.0S-OHMet, and 3.0S-Met groups (P < 0.05, Fig. 1). However, no significant differences were found in the vascular density of the placental stroma, or fold width and length per micrometer of the placenta (P > 0.05, Fig. 1).
Fig. 1.
Effect of increased consumption of methionine as OHMet on placental angiogenesis. Data were presented as means with standard error of the mean (SEM). Abbreviations: CON = basal diet; 1.5S-OHMet = basal diet + 1.5 g/kg OHMet; 3.0S-OHMet = basal diet + 3.0 g/kg OHMet; 3.0S-Met = basal diet + 3.0 g/kg Met. PSVD = the vessel density of placental stroma (× 40 magnification; scale bar, 200 μm). PFVD = the capillaries per unit area of placental fold (× 400 magnification; scale bar, 50 μm). Significant difference among the four treatments is marked with different superscripts (P < 0.05) (n = 6–8)
Effects of increased consumption of Met as OHMet on placental anti-oxidative capacity and inflammatory cytokines
To further investigate the impact of maternal Met supplementation on placental oxidative status, antioxidative parameters in the placenta were measured. As shown in Fig. 2, dietary 3.0S-Met consumption increased placental MDA levels (P < 0.05) compared to other groups and decreased placental GSH-Px activities (P = 0.05) when compared to the 3.0S-OHMet group. However, no significant differences were observed in the placental levels of T-SOD, CAT, iNOS, GSH and GSSG (P > 0.05). Furthermore, measurements of placental inflammatory cytokines revealed increased levels of IL-1β following consumption of 3.0S-OHMet or 3.0S-Met diets compared to the CON group (P < 0.05). Additionally, consumption of the 3.0S-OHMet diet increased placental IL-6 levels compared to both the CON and 1.5S-OHMet groups (P < 0.05). No differences were observed in placental TNF-α levels among the four treatment groups (P > 0.05).
Fig. 2.
Effect of increased consumption of methionine as OHMet on placental antioxidative capacity and inflammatory cytokines. GSH-Px, glutathione peroxidase; SOD, superoxide dismutase; CAT, catalase; MDA, malondialdehyde; iNOS, induced nitric oxide synthase; GSH, reduced glutathione; GSSG, oxidative glutathione. IL-6, interleukin 6; IL-1β, interleukin 1β; TNF-α, tumor necrosis factor α. Data were presented as means with standard error of the mean (SEM). Significant difference among the four treatments is marked with different superscripts (P < 0.05) (n = 6–8). Abbreviations: CON = basal diet; 1.5S-OHMet = basal diet + 1.5 g/kg OHMet; 3.0S-OHMet = basal diet + 3.0 g/kg OHMet; 3.0S-Met = basal diet + 3.0 g/kg Met
Placental proteomic analysis and identification of DEPs
A TMT-based quantitative proteomic analysis was performed to profile the expression of proteins and identify those related to placental function. A total of 5,135 proteins were quantified across all placentas (Table S2). Protein expression levels were normalized, resulting in the identification of 87 differentially expressed proteins (DEPs) (Table S3). Compared with the CON sows, 6 upregulated and 2 downregulated DEPs were found in the 1.5S-OHMet sows (Fig. 3A). Compared with the 3.0S-OHMet sows, 9 upregulated and 25 downregulated DEPs were found in the 1.5S-OHMet sows (Fig. 3B). Compared with the 3.0S-Met sows, 33 upregulated and 21 downregulated DEPs were found in the 1.5S-OHMet sows (Fig. 3C). Details of all differential proteins can be found in Table S3.
Fig. 3.
Effect of increased consumption of methionine as OHMet on placental proteins. A–C The cluster heat map and volcano map of differential proteins between CON and 1.5S-OHMet, 1.5S-OHMet and 3.0S-OHMet, and 1.5S-OHMet and 3.0S-Met. D Distribution of differential placental proteins in individual samples based on Pearson distance and average clustering. E–H Validation of four differentially expressed proteins from the results of mass spectrometry (MS) and western blotting (WB). TXN, thioredoxin; GSTT1, Glutathione transferase T1; HADHA, enoyl-CoA hydratase; NDUFA3, complex I-B9. The mean abundance of each protein is corrected to β-actin was used as a loading reference (n = 3). *P < 0.05, **P < 0.01. Abbreviations: CON = basal diet; 1.5S-OHMet = basal diet + 1.5 g/kg OHMet; 3.0S-OHMet = basal diet + 3.0 g/kg OHMet; 3.0S-Met = basal diet + 3.0 g/kg Met
Furthermore, 24 DEPs exhibited consistent changes across the three comparative analyses. These DEPs were identified and categorized into functional groups such as amino acid metabolism (2 DEPs), cellular apoptosis (2 DEPs), complement and coagulation cascade (4 DEPs), energy metabolism (3 DEPs), immune and inflammatory responses (3 DEPs), lipid metabolism (5 DEPs), oxidative status (4 DEPs) and signal transduction mechanisms (1 DEPs) (Fig. 3D and Table 2). Compared with the CON and 3.0S-OHMet groups, the 1.5S-OHMet group showed significantly upregulated glutathione-S-transferase (GSTT1). Compared with the 3.0S-OHMet and 3.0S-Met groups, the 1.5S-OHMet group showed significantly upregulated thioredoxin (TXN) and enolase 3 (ENO3), and significantly downregulated enoyl-CoA hydratase (HADHA), apo-lipoprotein B (APOB), 2,4-dienoyl-CoA reductase 1 (DECR1), galactocerebrosidase (GALC), complex I-B9 (NDUFA3), and hemoglobin subunit zeta (HBZ) in the placenta. Compared with the 3.0S-Met group, the 1.5S-OHMet group had significantly upregulated cathepsin B (CTSB), pro-cathepsin H (CTSH), and protein disulfide-isomerase (P4HB), and significantly downregulated C3/C5 convertase (CFB), bactericidal permeability-increasing protein (BPI), histone deacetylase 3 (HADC3) and TNF-α induced protein 8 (TNFAIP8) (Fig. 3D and Table 2).
Table 2.
Identification of differential expressed proteins in placenta
| Accession | Gene name | Description | CON vs. 1.5S-OHMet | 1.5S-OHMet vs. 3.0S-OHMet | 1.5S-OHMet vs. 3.0S-Met | Class | |||
|---|---|---|---|---|---|---|---|---|---|
| FC1 | P2 | FC1 | P2 | FC1 | P2 | ||||
| A0A5G2QYR2 | SAT2 | Thialysine N-epsilon-acetyltransferase | - | - | 1.26 | 0.023 | - | - | Amino acid metabolism |
| F1RLB2 | SPINK9 | Kazal-like domain-containing protein | - | - | 1.52 | 0.017 | 1.36 | 0.058 | Amino acid metabolism |
| A0A5S6GR81 | CTSB | Cathepsin B | - | - | - | - | 1.23 | 0.022 | Cellular apoptosis |
| A0A5G2QT38 | CTSH | Pro-cathepsin H | - | - | - | - | 1.22 | 0.049 | Cellular apoptosis |
| K7GPT9 | CFB | C3/C5 convertase | - | - | 0.71 | 0.237 | 0.80 | 0.029 | Complement and coagulation cascade |
| F1RGX7 | HBZ | Hemoglobin subunit zeta | - | - | 0.63 | 0.044 | 0.83 | 0.001 | Complement and coagulation cascade |
| A0A287A481 | PLG | Plasminogen | - | - | 0.71 | 0.025 | 0.84 | 0.081 | Complement and coagulation cascade |
| A0A5G2R920 | PROS1 | Vitamin K-dependent protein S | - | - | - | - | 0.81 | 0.025 | Complement and coagulation cascade |
| F1SGQ5 | ADCY4 | Adenylate cyclase type 4 | - | - | 0.80 | 0.008 | - | - | Energy metabolism |
| A0A5G2RCS9 | ENO3 | 2-phospho-D-glycerate hydrolyase | - | - | 1.22 | 0.070 | 1.29 | 0.026 | Energy metabolism |
| F1RNI5 | NDUFA3 | Complex I-B9 | - | - | 0.75 | 0.010 | 0.83 | 0.024 | Energy metabolism |
| A0A287BLV6 | BPI | Bactericidal permeability-increasing protein | - | - | - | - | 0.79 | 0.022 | Immune and inflammatory responses |
| F2Z4Z6 | HDAC3 | Histone deacetylase 3 | - | - | - | - | 0.78 | 0.009 | Immune and inflammatory responses |
| A0A287BL33 | TNFAIP8 | TNF alpha induced protein 8 | - | - | - | - | 0.83 | 0.004 | Immune and inflammatory responses |
| F1SCV9 | APOB | Vitellogenin domain-containing protein | - | - | 0.80 | 0.017 | 0.78 | 0.017 | Lipid metabolism |
| A0A5G2R5W7 | DECR1 | 2,4-dienoyl-CoA reductase 1 | - | - | - | - | 0.82 | 0.010 | Lipid metabolism |
| F1SDZ2 | GALC | Galactocerebrosidase | - | - | 0.82 | 0.073 | 0.79 | 0.003 | Lipid metabolism |
| A0A5G2R3M6 | HADHA | Enoyl-CoA hydratase | - | - | 0.88 | 0.007 | 0.84 | 0.026 | Lipid metabolism |
| I3LJL9 | MTMR8 | Phosphatidylinositol-3-phosphate phosphatase | - | - | 0.82 | 0.003 | 0.80 | 0.051 | Lipid metabolism |
| F1RL37 | GSTT1 | Glutathione transferase | 0.82 | 0.043 | 1.21 | 0.032 | - | - | Oxidative status |
| G9F6X8 | P4HB | Protein disulfide-isomerase | - | - | - | - | 1.38 | 0.014 | Oxidative status |
| A0A5G2QU43 | SDR16C5 | Short chain dehydrogenase/reductase family 16C member 5 | - | - | - | - | 0.75 | 0.031 | Oxidative status |
| A0A5G2Q9S8 | TXN | Thioredoxin | - | - | 1.27 | 0.045 | 1.47 | 0.035 | Oxidative status |
| F1RV28 | MAP2K6 | Mitogen-activated protein kinase 6 | - | - | 1.20 | 0.071 | 1.24 | 0.042 | Signal transduction mechanisms |
1FC (fold change) values were obtained from the mean peak area of the former sow/mean peak area of the latter sow. If the FC value is greater than 1, it indicates that the protein level is higher in the former group than in the latter group
2P-values were calculated from the student’s t-test with a threshold of 0.05
Four DEPs were chosen to validate the proteomic results by western blotting (Fig. 3E–H). TXN was downregulated and NUDFA3 was upregulated in both 3.0S-OHMet and 3.0S-Met groups compared with the 1.5S-OHMet group. Moreover, GSTT1 was upregulated in the 1.5S-OHMet group compared with both the CON and 3.0S-OHMet groups. Additionally, HADHA was upregulated in the 3.0S-OHMet and 3.0S-Met groups compared with the 1.5S-OHMet group.
GO and KEGG enrichment analyses of DEPs
GO enrichment and KEGG pathway analysis were performed to explore the molecular mechanisms underlying the differential expression of proteins involved in placental function (Fig. 4 and Table S4, S5). In the comparison between 1.5S-OHMet and CON placentas, the most enriched GO pathways were “transport”, “cytoplasm” and “nucleus” (Fig. 4A1), and the enriched KEGG pathways included “glutathione metabolism” and “protein export” (Fig. 4B1). For the placental DEPs in 1.5S-OHMet compared to 3.0S-OHMet placentas, the most enriched GO pathways were “extra-celluar region”, “cytoplasm” and “membrane” (Fig. 4A2), while the enriched KEGG pathways included “complement and coagulation cascades” and “glutathione metabolism” (Fig. 4B2). For the placental DEPs in 1.5S-OHMet compared to 3.0S-Met placentas, the most enriched GO pathways were “cytoplasm”, “nucleus” and “metal ion binding” (Fig. 4A3); while the enriched pathways of KEGG included “NOD-like receptor signaling pathway”, “retinol metabolism” and “apoptosis” (Fig. 4B3).
Fig. 4.
Functional characterization of differentially expressed proteins. A1–A3 GO enrichment analysis of differentially expressed proteins, based on the categories of biological process, cellular component, and molecular function, between CON and 1.5S-OHMet, 1.5S-OHMet and 3.0S-OHMet, 1.5S-OHMet and 3.0S-Met, respectively. B1–B3 The KEGG pathway of differential proteins between CON and 1.5S-OHMet, 1.5S-OHMet and 3.0S-OHMet, 1.5S-OHMet and 3.0S-Met, respectively. Abbreviations: CON = basal diet; 1.5S-OHMet = basal diet + 1.5 g/kg OHMet; 3.0S-OHMet = basal diet + 3.0 g/kg OHMet; 3.0S-Met = basal diet + 3.0 g/kg Met
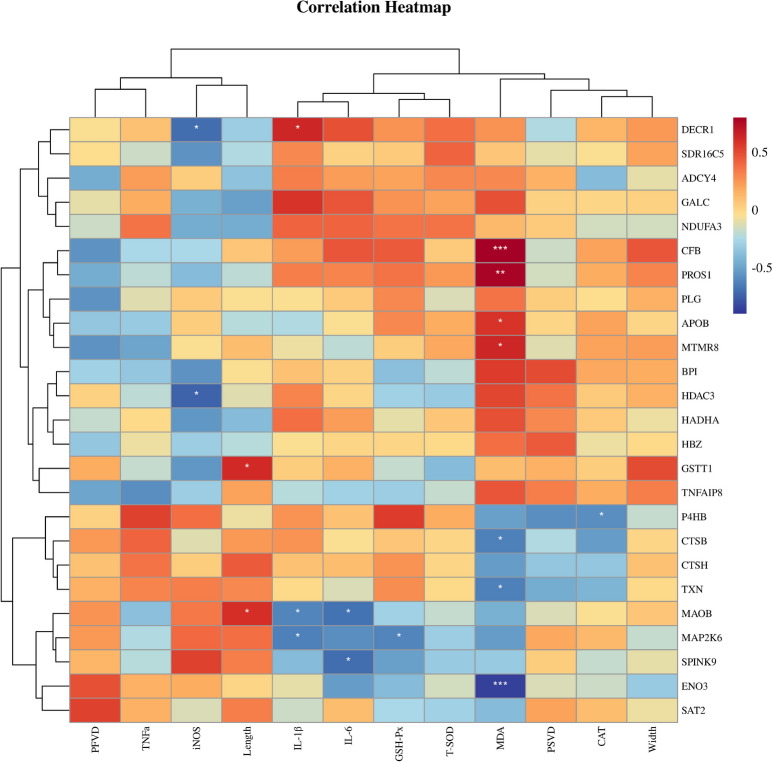
Association between the differential proteins and placental traits
As shown in Fig. 5, antioxidative indexes determined that the placental MDA levels exhibited negative correlations with the changes in ENO3, TXN and CTSB, and positive correlations with the changes in CFB, PROS1 APOB and MTMR8. Additionally, inflammation indexes revealed that the IL-1β levels were negatively correlated with changes in amine oxidase (MAOB) and mitogen-activated protein kinase 6 (MAP2K6), and positively correlated with changes in DECR1. Moreover, the IL-6 levels were negatively correlated with changes in MAOB and Kazal-type protein 1 (SPINK).
Fig. 5.
The correlation of placental differential proteins and placental indexes. PSVD, density vascular of placental stroma; PFVD, capillaries per unit area of the fold; Width, fold width; Length, placental fold length; MDA, malondialdehyde; GSH-Px, activity of glutathione peroxidase; SOD, superoxide dismutase; CAT, catalase; iNOS, inducible nitric oxide synthase; TNFα, tumor necrosis factor α; IL-6, interleukin 6; IL-1β, interleukin 1β. *P < 0.05, **P < 0.01, ***P < 0.001
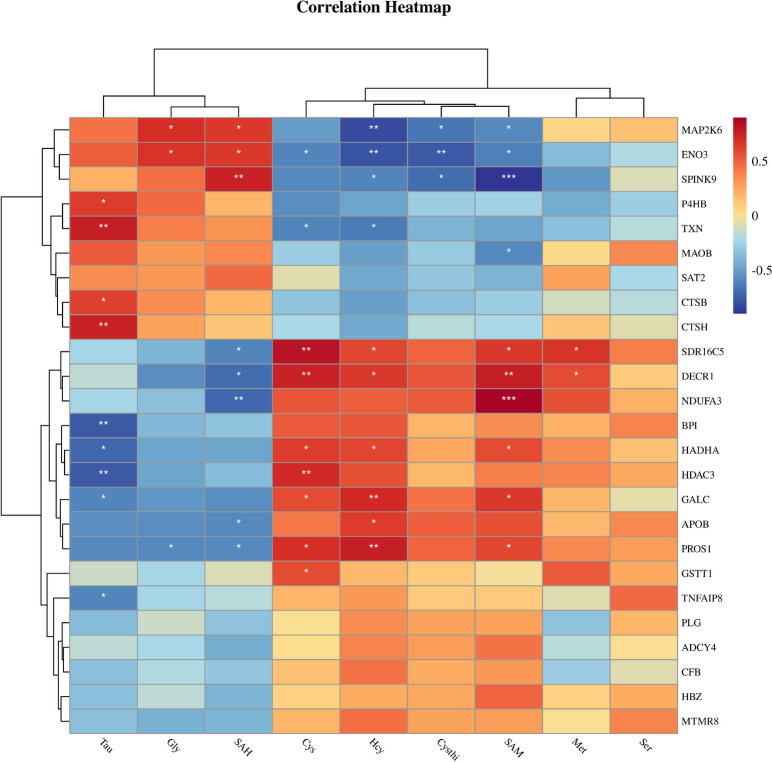
Association between the differential proteins and maternal methionine metabolites
To investigate the relationship between Met metabolites and DEPs, heat maps were generated by Pearson correlation analysis (Fig. 6). The Met metabolites data were obtained from our previous study [17]. Hcy levels were negatively correlated with changes in TXN and ENO3, and positively correlated with changes in HADHA, APOB, DECR1, and GALC. Cys levels were positively correlated with changes in GSTT1. In addition, SAM levels were positively correlated with changes in NDUFA3, and negatively correlated with changes in SPINK9. Tau levels were positively correlated with changes in TXN and P4HB, and negatively correlated with changes in HADHA, HDAC3, BPI, TNFAIP8, and GALC3.
Fig. 6.
The correlation of placental differential proteins and maternal methionine metabolites. Met, methionine; SAM, S-adenosyl-methionine; SAH, S-adenosyl-homocysteine; Hcy, homocysteine; Cysthi, cystathionine; Tau, taurine; Ser, serine; Gly, glycine. *P < 0.05, **P < 0.01, ***P < 0.001
Increased consumption of methionine as OHMet improved angiogenesis and antioxidative capacity in vitro
To confirm the improved antioxidative capacity and angiogenesis from maternal supplementation with 1.5S-OHMet, pTr cells were cultured using maternal serum from each treatment (Fig. 7A). Serum from the 1.5S-OHMet group significantly increased pTr cell viability and decreased ROS levels compared with other groups (Fig. 7B and C). Furthermore, serum from the 1.5S-OHMet group upregulated the gene expression of Mat2b, Dnmta, Dnmt3b, and Cth compared with other groups, and also upregulated the gene expression of Cbs, Gstt1, Ang2, and Igf2r compared with the 3.0S-OHMet and 3.0S-Met groups (Fig. 7E–G). Serum from the 3.0S-OHMet group upregulated the gene expression of Cth and Cbs compared with the 3.0S-Met group (Fig. 7E). Serum from the 1.5S-OHMet group upregulated the gene expression of Vegf-a compared with the 3.0S-Met group (Fig. 7G). As shown in Fig. 7H, serum from the 1.5S-OHMet group upregulated the protein expression of GSTT1 and TXN compared with other groups, and upregulated the protein expression of VEGF-A compared with the 3.0S-OHMet group.
Fig. 7.
Increased consumption of methionine as OHMet improved angiogenesis and antioxidative capacity in pTr cells. A The trophoblast cells were treated for 24 h with 20% serum from each group (n = 3). B Cell viability. C Reactive oxidative species level. D Schematic of the Met metabolism and list of genes directly involved and associated with this pathway. E The gene expression of transmethylation and trans-sulphuration. F The gene expression of anti-oxidation. G The gene expression of angiogenesis. E–G Duplicated independent experiments. H The protein expression of pTr, GAPDH as a loading control. TXN, thioredoxin; GSTT1, glutathione transferase T1; VEGF-A, vascular endothelial growth factor A. Significant difference among four treatments is marked with different superscripts (P < 0.05). Abbreviations: CON = basal diet; 1.5S-OHMet = basal diet + 1.5 g/kg OHMet; 3.0S-OHMet = basal diet + 3.0 g/kg OHMet; 3.0S-Met = basal diet + 3.0 g/kg Met
Hcy induced anti-proliferative effects and reduced antioxidant capacity of pTr cells
To further investigate the effect of Hcy on the proliferation and antioxidant capacity of pTr cells, various concentrations of Hcy were administered to the pTr cells. Compared to the 0 μmol/L Hcy group, the release of LDH was observed in pTr cells at Hcy concentrations of 200, 400, and 800 μmol/L (Fig. 8A). Moreover, treatment of pTr cells with 800 μmol/L Hcy significantly reduced cell proliferation (Fig. 8B and C). Additionally, cells treated with 200, 400, and 800 μmol/L Hcy had impaired cell migration and increased ROS levels (Fig. 8D and E). The protein expression of ENO3 was significantly inhibited when pTr cells were treated with Hcy concentrations of 200, 400, and 800 μmol/L (Fig. 8F). The protein expression of TXN was significantly inhibited at 800 μmol/L Hcy compared with the 200 μmol/L Hcy group (Fig. 8F). Finally, Hcy was found to increase the percentage of apoptosis in pTr cells at 24 and 48 h (Fig. 8G–J).
Fig. 8.
Anti-proliferative and inhibited antioxidant capacity induced by homocysteine in pTr cells (n = 3). A Detection of LDH activity in pTr cells. B EdU-positive cells were measured using ImageJ software. C Cell proliferation was determined by the EdU assay, and images were taken under a fluorescence microscope (magnification 400 × , bar = 100 μm). D Cell migration was measured using ImageJ software. E The cell migration distance (bar = 500 μm) and images of ROS were taken using a DCFH-DA probe (magnification 400 × , bar = 500 μm). F Representative western blot results for protein levels of enolase 3 (ENO3) and thioredoxin (TXN), β-actin was used as a loading reference. G–J Detection of apoptosis in pTr cells. Annexin V and propidium iodide (PI) fluorescence was quantified using flow cytometry. *P < 0.05, **P < 0.01, ***P < 0.001
Discussion
The placenta is responsible for nutritional support and exchange between the mother and fetus, directly affecting fetal growth and development [23]. Previous studies have shown that Met metabolism during late pregnancy noticeably influences both placental vessel density and antioxidant capacity [6]. Growing evidence suggests that Met supplementation can improve the reproductive performance of sows by promoting maternal metabolism and enhancing placental function [8, 10]. However, the mechanisms by which Met nutrition improves placental development are unclear. Thus, this study aimed to explore how various levels and sources of Met regulate placental function.
Consumption of 1.5S-OHMet improved placental angiogenesis by enhancing antioxidant capacity
In the current study, sows supplemented with 1.5S-OHMet exhibited the highest number of capillaries per unit area of placental fold, aligning with previous findings that maternal dietary Met supplementation during late gestation enhances placental angiogenesis [6]. Furthermore, serum from the 1.5S-OHMet group upregulated the mRNA expression of Ang2 and Vegf-α in pTr cells and increased the protein expression of VEGF-A. This upregulation of VEGF-A facilitated angiogenesis by its interaction with VEGFR [2]. Notably, the KEGG enrichment analysis of DEPs identified pathways linked to antioxidant capacity. Sows on 1.5S-OHMet diets exhibited upregulated protein expression of GSTT1 and TXN in both placenta and pTr cells, indicating an enhanced antioxidant capacity. This finding is consistent with our earlier work, which showed that increased maternal intake of OHMet improved the antioxidant capacity of the neonatal intestine [24]. Overall, these results suggest that maternal supplementation with 1.5S-OHMet improves placental angiogenesis and oxidative status.
Excessive Met consumption reduced placental antioxidant capacity via accumulating Hcy
Our previous study indicated a significant rise in Hcy levels as pregnancy progressed, with excessive Met consumption exacerbating Hcy accumulation [17]. Given that Hcy is known to impact early extra-embryonic angiogenesis by reducing antioxidant capacity [25], we suggest that the placental oxidative stress in the 3.0S-Met group may be attributed to elevated maternal Hcy levels. Previous studies have shown that high Hcy concentration can lead to the generation of ROS, downregulation of TXN, increased MDA levels, inhibition of iNOS activity, and ultimately contribute to oxidative stress and cell apoptosis [12, 26, 27]. Consistent with our findings, the 3.0S-Met group showed increased placental MDA levels and apoptosis-related CTSB protein expression, along with decreased TXN protein expression, compared to the 1.5S-OHMet group. Furthermore, we observed a significant negative correlation between maternal Hcy levels and placental TXN expression, corroborating earlier studies [28]. Further studies confirmed that elevated Hcy concentrations inhibit TXN expression and increase ROS levels and the rate of apoptosis in pTr cells. Furthermore, we observed a negative correlation between placental MDA levels and placental TXN expression. MDA, a byproduct of lipid peroxidation, serves as an indicator of oxidative stress [29]. These results suggest that elevated Hcy levels lead to increased MDA levels and decreased TXN expression in the placenta, thus reducing its antioxidant capacity.
Further analysis revealed elevated expression of placental APOB, DECR1, and HADHA proteins in sows fed diets containing 3.0S-OHMet or 3.0S-Met compared to those fed 1.5S-OHMet. DECR1 and HADHA are recognized for their roles in the beta-oxidation pathway of fatty acids, whereas APOB is implicated in lipid transport [30, 31]. We propose that placentas from sows on 3.0S-OHMet and 3.0S-Met diets experience greater lipid catabolism compared to those on 1.5S-OHMet diets. This suggests that the 3.0S-Met diets are associated with lipid peroxidation. Moreover, the increased MDA levels in placentas from sows on 3.0S-Met diets indicate lipid peroxidation. Similar findings were reported by Zhou et al., which indicated that ectopic lipid accumulation can lead to lipotoxicity in the placenta [32].
Studies have shown that maternal obesity can lead to placental lipotoxicity, which induces inflammation and oxidative stress [33]. Consistent with the current study, sows fed the 1.5S-OHMet diet exhibited downregulated placental TNFAIP8 protein expression and upregulated P4HB expression compared to those fed the 3.0S-Met diet. TNFAIP8 binds to fatty acids, regulates the expression of enzymes involved in lipid and fatty acid metabolism, and modulates autophagy and cellular steatosis [34]. Furthermore, TXN and protein disulfide isomerase (P4HB), components of the thioredoxin system, play essential roles in modulating oxidative stress [35]. These results suggested that maternal consumption of the 1.5S-OHMet diet reduced the oxidation of lipid metabolites by enhancing antioxidant capacity, whereas 3.0S-Met supplementation may disrupt placental lipid metabolism.
Furthermore, maternal 3.0S-OHMet or 3.0S-Met diet consumption increased placental IL-1β levels. Sows fed a 3.0S-Met diet had upregulated placental HDAC3 expression compared with those fed CON or 1.5S-OHMet diet. Oxidative stress activates NF-κB via ROS production, resulting in the production of IL-1β and IL-6, and subsequent inflammation [36, 37]. Additionally, HDAC3 promotes acute inflammation induced by lipopolysaccharide by enhancing NLRP3-dependent caspase-1 activation [38]. In our previous study, sows fed 3.0S-Met had decreased serum butyric acid levels compared to those fed 1.5S-OHMet [17]. Butyric acid is reported to mitigate tissue inflammation by inhibiting HDAC3 expression and prevent oxidative stress by reducing ROS production [39]. These results suggest that the reduced butyric acid level may be one cause of placental inflammation. Moreover, placentas from dietary supplementation with 1.5S-OHMet exhibited lower PLG expression and lower CFB expression compared to those from the 3.0S-OHMet or 3.0S-Met groups. Upregulation of CFB promotes the activation of the C3 convertase enzyme [40]. PLG binds to C5 and C3 resulting in increased levels of C4b-binding protein, suggesting potential interactions during acute inflammation [41]. These results indicate that 1.5S-OHMet supplementation improves lipid metabolism and reduces inflammation, whereas excessive Met supplementation may induce placental inflammation.
Excessive Met consumption affects placental energy metabolism via accumulating Hcy
Maternal nutrition influences placental function, modulating energy metabolism [42]. Ensuring adequate energy supplementation for sows during farrowing is essential for preventing fetal mortality [43]. Analysis of differential protein expression in the placenta revealed that sows fed diets containing 3.0S-Met or 3.0S-OHMet had decreased levels of ENO3 protein and increased levels of NDUFA3 protein compared to those fed 1.5S-OHMet. Notably, the ENO3 protein participated in the pathway synthesizing pyruvate from D-glyceraldehyde 3-phosphate [44]. Downregulation of the ENO3 protein in placentas from sows fed 3.0S-Met and 3.0S-OHMet diets suggests a potential reduction in phosphoenolpyruvate synthesis. Furthermore, NDUFA3, a component of Complex I, plays a role in generating ROS during oxidative phosphorylation [45]. In our previous study, sows fed 3.0S-Met and 3.0S-OHMet diets had higher succinate levels than those fed 1.5S-OHMet diet [17]. Therefore, we hypothesize that an inadequate energy supply may have led to the compensatory upregulation of NDUFA3 in placentas of sows fed the 3.0S-Met and 3.0S-OHMet diets. Additional experiments demonstrated that increased Hcy levels significantly suppressed ENO3 expression in pTr cells, confirming that excessive maternal Met supplementation can disrupt energy metabolism and impair placental function.
OHMet resulted in lower accumulation of Hcy compared to Met
During late gestation, significant changes in maternal Met metabolites occurred [10], impacting maternal glycolipid and amino acid metabolism [17]. To further explore the alterations in placental Met metabolism induced by difference in Met nutrition, the gene expression of the transmethylation and trans-sulphuration pathways in pTr cells was measured. Intriguingly, serum from the 1.5S-OHMet group increased the mRNA levels of Mat2b, Dnmt3a, and Dnmt3b in vitro, indicating that 1.5S-OHMet promote the transmethylation pathway. Expression of MAT2B was increased in the OHMet group, which facilitates SAM biosynthesis [46]. The increased expression of DNMT3A and DNMT3B may suggest that OHMet improves placental methylation levels [47]. Additionally, a previous study suggested that OHMet could promote the transsulfuration of Met [48]. In line with our findings, the expression of Cbs and Cth genes in the 3.0S-OHMet group was significantly higher than those in the 3.0S-Met group. These findings suggest that OHMet results in lower Hcy accumulation than crystalline Met through the trans-sulphuration pathway [49].
Our research had limitations. Firstly, it was unclear whether the improvement in placental function was achieved through OHMet itself or its metabolites. Secondly, the optimal dosage of OHMet was not determined and further research is warranted to determine the dosage for improving the reproductive performance of gestation sows.
Conclusion
Maternal supplementation with 1.5S-OHMet improved placental development by promoting angiogenesis and enhancing antioxidative capacity. In contrast, consumption of 3.0S-OHMet or 3.0S-Met diets resulted in increased placental inflammatory cytokine levels, disturbed lipid metabolism, and altered oxidative status, attributed to increased Hcy levels. These findings underscore the importance of optimizing maternal Met intake levels and sources during late pregnancy to enhance placental function.
Supplementary Information
Additional file 1: Fig. S1. The protein detection for placental samples.
Additional file 2: Table S1. Primers used for quantitative real-time PCR (qPCR). Table S2. A total of 5,136 proteins were quantified from all the placentas. Table S3. Differentially abundant proteins (DAPs) identified in the placentas of sows from the CON vs. 1.5S-OHMet, 1.5S-OHMet vs. 3.0S-OHMet and 1.5S-OHMet vs. 3.0S-Met groups. Table S4. Significantly enriched KEGG pathway of the differentially abundant proteins (DAPs). Table S5. Significantly enriched GO Slim terms of the differentially abundant proteins (DAPs).
Acknowledgements
The authors would like to thank the staff of the Key Laboratory for Animal Disease Resistance Nutrition of the Ministry of Education for their ongoing assistance.
Abbreviations
- APOB
Apo-lipoprotein B
- BPI
Bactericidal permeability-increasing protein
- CAT
Catalase
- CTSB
Cathepsin B
- CTSH
Pro-cathepsin H
- CFB
C3/C5 convertase
- DECR1
2,4-Dienoyl-CoA reductase 1
- ENO3
Enolase 3
- FW
Fold width
- GALC
Galactocerebrosidase
- GSH-Px
Glutathione peroxidase
- GSH
Reduced glutathione
- GSSG
Oxidative glutathione
- Hcy
Homocysteine
- HADHA
Enoyl-CoA hydratase
- HBZ
Hemoglobin subunit zeta
- HADC3
Histone deacetylase 3
- iNOS
Inducible nitric oxide synthase
- IL-1β
Interleukin 1β
- IL-6
Interleukin 6
- MDA
Malondialdehyde
- NDUFA3
Complex I-B9
- SOD
Superoxide dismutase
- SAM
S-adenosyl-methionine
- SAH
S-adenosyl-homocysteine
- PSVD
Density vascular of placental stroma
- PFVD
Capillaries per unit area of the fold
- P4HB
Protein disulfide-isomerase
- PFL
Placental fold length
- TXN
Thioredoxin
- TNFAIP8
TNF-α induced protein 8
- VEGF-A
Vascular endothelial growth factor A
Authors’ contributions
RZ, SL and LZ carried out the animal rearing trial, and did the laboratory work and data statistical analysis, RZ, YM and ZF wrote the manuscript, PY, SL, YM, LY, LH, XZ, HL, YZ, SX, YL, LC, BF and DW participated in the design of the experiment and the editing of the manuscript, ZF conceived and supervised the experiment. All authors have read the manuscript and agree to publish the version of the manuscript.
Funding
This study was financially supported by the Adisseo Innovation Research Center for Nutrition and Health (060–2222319005), Natural Science Foundation of Sichuan (2023NSFSC1135) and National Natural Science Foundation of China (31972603).
Data availability
The datasets analyzed in the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The experimental scheme was approved by the Institutional Animal Care and Use Committee of Sichuan Agricultural University (Ya’an, China) (approval No. SYXK-Chuan-2014-184).
Consent for publication
Not applicable.
Competing interests
The financial support was partially from Adisseo, the producer of OHMet. And Yves Mercier is the member of Adisseo. However, this does not alter our adherence to Animal Nutrition policies on sharing data and materials. All authors agreed to publish this paper.
References
- 1.Huang S, Wu Z, Huang Z, Hao X, Zhang L, Hu C, et al. Maternal supply of cysteamine alleviates oxidative stress and enhances angiogenesis in porcine placenta. J Anim Sci Biotechnol. 2021;12:91. 10.1186/s40104-021-00609-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu C, Wu Z, Huang Z, Hao X, Wang S, Deng J, et al. Nox2 impairs VEGF-A-induced angiogenesis in placenta via mitochondrial ROS-STAT3 pathway. Redox Biol. 2021;45:102051. 10.1016/j.redox.2021.102051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu D, Feng L, Hao X, Huang S, Wu Z, Ma S, et al. Effects of dietary supplementation of gestating sows with adenosine 5’-monophosphate or adenosine on placental angiogenesis and vitality of their offspring. J Anim Sci. 2022;100:skac237. 10.1093/jas/skac237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bai J, Li J, Liu N, Jia H, Si X, Zhou Y, et al. Glucosamine alleviates zearalenone-induced damage to porcine trophectoderm cells by activating the PI3K/AKT signaling pathway. Food Funct. 2022;13:7857–70. 10.1039/d2fo00928e. [DOI] [PubMed] [Google Scholar]
- 5.Tan C, Huang Z, Xiong W, Ye H, Deng J, Yin Y. A review of the amino acid metabolism in placental function response to fetal loss and low birth weight in pigs. J Anim Sci Biotechnol. 2022;13:28. 10.1186/s40104-022-00676-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xia M, Pan Y, Guo LL, Wei XWX, Xiong J, Wang L, et al. Effect of gestation dietary methionine/lysine ratio on placental angiogenesis and reproductive performance of sows. J Anim Sci. 2019;97:3487–97. 10.1093/jas/skz175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Research Council. Nutrient Requirements of Swine. 11th revised ed. Washington (DC): National Academic Press, 2012.
- 8.Bin P, Azad MA, Liu G, Zhu D, Kim SW, Yin YL. Effects of different levels of methionine on sow health and plasma metabolomics during late gestation. Food Funct. 2018;9:4979–88. 10.1039/c8fo01477a. [DOI] [PubMed] [Google Scholar]
- 9.Azad MAK, Bin P, Liu G, Fang J, Li T, Yin Y. Effects of different methionine levels on offspring piglets during late gestation and lactation. Food Funct. 2018;9:5843–54. 10.1039/c8fo01343h. [DOI] [PubMed] [Google Scholar]
- 10.Xia M, Peng J, Cui C, Gu Q, Zhou L, Wang C, et al. Effect of gestation dietary methionine-to-lysine ratio on methionine metabolism and antioxidant ability of high-prolific sows. Anim Nutri. 2021;7:849–58. 10.1016/j.aninu.2021.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou R, Zhe L, Chen F, Gao T, Zhang X, Huang L, et al. Maternal folic acid and vitamin B12 supplementation during medium to late gestation promotes fetal development via improving placental antioxidant capacity, angiogenesis and amino acid transport. J Sci Food Agric. 2024;104:2832–41. 10.1002/jsfa.13171. [DOI] [PubMed] [Google Scholar]
- 12.Wei H, Zhao X, Xia M, Tan C, Gao J, Htoo JK, et al. Different dietary methionine to lysine ratios in the lactation diet: effects on the performance of sows and their offspring and methionine metabolism in lactating sows. J Anim Sci Biotechnol. 2019;10:76. 10.1186/s40104-019-0373-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie M, Hou SS, Huang W, Fan HP. Effect of excess methionine and methionine hydroxy analogue on growth performance and plasma homocysteine of growing Pekin ducks. Poult Sci. 2007;86:1995–9. 10.1093/ps/86.9.1995. [DOI] [PubMed] [Google Scholar]
- 14.Li Q, Yang S, Chen F, Guan W, Zhang S. Nutritional strategies to alleviate oxidative stress in sows. Anim Nutr. 2022;9:60–73. 10.1016/j.aninu.2021.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee C, Oh J, Hristov AN, Harvatine K, Vazquez-Anon M, Zanton GI. Effect of 2-hydroxy-4-methylthio-butanoic acid on ruminal fermentation, bacterial distribution, digestibility, and performance of lactating dairy cows. J Dairy Sci. 2015;98:1234–47. 10.3168/jds.2014-8904. [DOI] [PubMed] [Google Scholar]
- 16.Fang Z, Luo H, Wei H, Huang F, Qi Z, Jiang S, et al. Methionine metabolism in piglets Fed DL-methionine or its hydroxy analogue was affected by distribution of enzymes oxidizing these sources to keto-methionine. J Agric Food Chem. 2010;58:2008–14. 10.1021/jf903317x. [DOI] [PubMed] [Google Scholar]
- 17.Zhou R, Zhe L, Mercier Y, Hu L, Li R, Chen H, et al. Serum metabolomics analysis reveals a novel association of pregnant metabolism and fetal survival in sows with consumption of methionine levels and sources. Anim Nutr. 2025;20:145–57. 10.1016/j.aninu.2024.07.008. [DOI] [PMC free article] [PubMed]
- 18.Vallet J, Freking B. Differences in placental structure during gestation associated with large and small pig fetuses. J Anim Sci. 2007;85:3267–75. 10.2527/jas.2007-0368. [DOI] [PubMed] [Google Scholar]
- 19.Peng X, Huang Y, Wang G, He Y, Hu L, Fang Z, et al. Maternal long-term intake of inulin improves fetal development through gut microbiota and related metabolites in a rat model. J Agric Food Chem. 2022;70:1840–51. 10.1021/acs.jafc.1c07284. [DOI] [PubMed] [Google Scholar]
- 20.Zhao X, Jia W, Wang J, Wang S, Zheng Q, Shan T. Identification of a candidate gene regulating intramuscular fat content in pigs through the integrative analysis of transcriptomics and proteomics data. J Agric Food Chem. 2023;71:19154–64. 10.1021/acs.jafc.3c05806. [DOI] [PubMed] [Google Scholar]
- 21.Milona A, Owen BM, Cobbold JF, Willemsen EC, Cox IJ, Boudjelal M, et al. Raised hepatic bile acid concentrations during pregnancy in mice are associated with reduced farnesoid X receptor function. Hepatology. 2010;52:1341–9. 10.1002/hep.23849. [DOI] [PubMed] [Google Scholar]
- 22.Lin S, Yang X, Yuan P, Yang J, Wang P, Zhong H, et al. Undernutrition shapes the gut microbiota and bile acid profile in association with altered gut-liver FXR signaling in weaning pigs. J Agric Food Chem. 2019;67:3691–701. 10.1021/acs.jafc.9b01332. [DOI] [PubMed] [Google Scholar]
- 23.Barbaux S, Erwich JJHM, Favaron P, Gil S, Gallot D, Golos TG, et al. IFPA meeting 2014 workshop report: Animal models to study pregnancy pathologies; new approaches to study human placental exposure to xenobiotics; biomarkers of pregnancy pathologies; placental genetics and epigenetics; the placenta and stillbirth and fetal growth restriction. Placenta. 2015;36 Suppl:S5–10. 10.1016/j.placenta.2015.01.196. [DOI] [PubMed] [Google Scholar]
- 24.Zhong H, Li H, Liu G, Wan H, Mercier Y, Zhang X, et al. Increased maternal consumption of methionine as its hydroxyl analog promoted neonatal intestinal growth without compromising maternal energy homeostasis. J Anim Sci Biotechnol. 2016;7:46. 10.1186/s40104-016-0103-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oosterbaan AM, Steegers EA, Ursem NT. The effects of homocysteine and folic acid on angiogenesis and VEGF expression during chicken vascular development. Microvasc Res. 2012;83:98–104. 10.1016/j.mvr.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 26.Tyagi N, Sedoris KC, Steed M, Ovechkin AV, Moshal KS, Tyagi SC. Mechanisms of homocysteine-induced oxidative stress. Am J Physiol-Heart C. 2005;289:H2649–56. 10.1152/ajpheart.00548.2005. [DOI] [PubMed] [Google Scholar]
- 27.Kamudhamas A, Pang L, Smith SD, Sadovsky Y, Nelson DM. Homocysteine thiolactone induces apoptosis in cultured human trophoblasts: a mechanism for homocysteine-mediated placental dysfunction? Am J Obstet Gynecol. 2004;191:563–71. 10.1016/j.ajog.2004.01.037. [DOI] [PubMed] [Google Scholar]
- 28.Wu Y, Yang L, Zhong L. Decreased serum levels of thioredoxin in patients with coronary artery disease plus hyperhomocysteinemia is strongly associated with the disease severity. Atherosclerosis. 2010;212:351–5. 10.1016/j.atherosclerosis.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 29.Ayala A, Muñoz MF, Argüelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev. 2014;2014:360438. 10.1155/2014/360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nassar ZD, Mah CY, Dehairs J, Burvenich IJ, Irani S, Centenera MM, et al. Human DECR1 is an androgen-repressed survival factor that regulates PUFA oxidation to protect prostate tumor cells from ferroptosis. Elife. 2020;9:e54166. 10.7554/elife.54166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thapa D, Wu K, Stoner MW, Xie B, Zhang M, Manning JR, et al. The protein acetylase GCN5L1 modulates hepatic fatty acid oxidation activity via acetylation of the mitochondrial β-oxidation enzyme HADHA. J Biol Chem. 2018;293:17676–84. 10.1074/jbc.ac118.005462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou Y, Xu T, Cai A, Wu Y, Wei H, Jiang S, et al. Excessive backfat of sows at 109 d of gestation induces lipotoxic placental environment and is associated with declining reproductive performance. J Anim Sci. 2018;96:250–7. 10.1093/jas/skx041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oliva K, Barker G, Riley C, Bailey MJ, Permezel M, Rice GE, et al. The effect of pre-existing maternal obesity on the placental proteome: two-dimensional difference gel electrophoresis coupled with mass spectrometry. J Mol Endocrinol. 2012;48:139–49. 10.1530/jme-11-0123. [DOI] [PubMed] [Google Scholar]
- 34.Niture S, Gyamfi MA, Lin M, Chimeh U, Dong X, Zheng W, et al. TNFAIP8 regulates autophagy, cell steatosis, and promotes hepatocellular carcinoma cell proliferation. Cell Death Dis. 2020;11:178. 10.1038/s41419-020-2369-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Espinosa B, Arnér ES. Thioredoxin-related protein of 14 kDa as a modulator of redox signalling pathways. Br J Pharmacol. 2019;176:544–53. 10.1111/bph.14479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee YM, Song BC, Yeum KJ. Impact of volatile anesthetics on oxidative stress and inflammation. BioMed Res Int. 2015;2015:242709. 10.1155/2015/242709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morgan MJ, Liu ZG. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011;21:103–15. 10.1038/cr.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chi Z, Chen S, Xu T, Zhen W, Yu W, Jiang D, et al. Histone deacetylase 3 couples mitochondria to drive il-1β-dependent inflammation by configuring fatty acid oxidation. Mol Cell. 2020;80:43–58.e7. 10.1016/j.molcel.2020.08.015. [DOI] [PubMed] [Google Scholar]
- 39.Schulthess J, Pandey S, Capitani M, Rue-Albrecht KC, Arnold I, Franchini F, et al. The short chain fatty acid butyrate imprints an antimicrobial program in macrophages. Immunity. 2019;50(2):432–45. e7. 10.1016/j.immuni.2018.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang P, Song Y, Zhong H, Lin S, Zhang X, Li J, et al. Transcriptome profiling of placenta through pregnancy reveals dysregulation of bile acids transport and detoxification function. Int J Mol Sci. 2019;20:4099. 10.3390/ijms20174099. [DOI] [PMC free article] [PubMed]
- 41.Baker SK, Strickland S. A critical role for plasminogen in inflammation. J Exp Med. 2020;217:e20191865. 10.1084/jem.20191865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Che L, Yang Z, Xu M, Xu S, Che L, Lin Y, et al. Maternal nutrition modulates fetal development by inducing placental efficiency changes in gilts. BMC Genomics. 2017;18:213. 10.1186/s12864-017-3601-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nielsen SE, Feyera T, Skovmose SJW, Krogh U, Eskildsen M, Theil PK. Intravenous infusion of glucose improved farrowing performance of hyperprolific crossbred sows. J Anim Sci. 2021;99:skab161. 10.1093/jas/skab061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cui H, Guo D, Zhang X, Zhu Y, Wang Z, Jin Y, et al. ENO3 inhibits growth and metastasis of hepatocellular carcinoma via Wnt/β-catenin signaling pathway. Front Cell Dev Biol. 2021;9: 797102. 10.3389/fcell.2021.797102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rak M, Rustin P. Supernumerary subunits NDUFA3, NDUFA5 and NDUFA12 are required for the formation of the extramembrane arm of human mitochondrial complex I. FEBS Lett. 2014;588:1832–8. 10.1016/j.febslet.2014.03.046. [DOI] [PubMed] [Google Scholar]
- 46.Zuo F, Gu Q, Li S, Wei H, Peng J. Effects of different methionine sources on methionine metabolism in the IPEC-J2 cells. BioMed Res Int. 2019;2019:5464906. 10.1155/2019/5464906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Andrews S, Krueger C, Mellado-Lopez M, Hemberger M, Dean W, Perez-Garcia V, et al. Mechanisms and function of de novo DNA methylation in placental development reveals an essential role for DNMT3B. Nat Commun. 2023;14:371. 10.1038/s41467-023-36019-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zuo F, Wei H, Peng J, Li S, Zhou Y. Effects on the cell barrier function of L-Met and DL-HMTBA Is related to metabolic characteristics and m6A modification. Front Nutr. 2022;9:836069. 10.3389/fnut.2022.836069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang X, Li H, Liu G, Wan H, Mercier Y, Wu C, et al. Differences in plasma metabolomics between sows fed DL-methionine and its hydroxy analogue reveal a strong association of milk composition and neonatal growth with maternal methionine nutrition. Br J Nutr. 2015;113:585–95. 10.1017/s0007114514004036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Fig. S1. The protein detection for placental samples.
Additional file 2: Table S1. Primers used for quantitative real-time PCR (qPCR). Table S2. A total of 5,136 proteins were quantified from all the placentas. Table S3. Differentially abundant proteins (DAPs) identified in the placentas of sows from the CON vs. 1.5S-OHMet, 1.5S-OHMet vs. 3.0S-OHMet and 1.5S-OHMet vs. 3.0S-Met groups. Table S4. Significantly enriched KEGG pathway of the differentially abundant proteins (DAPs). Table S5. Significantly enriched GO Slim terms of the differentially abundant proteins (DAPs).
Data Availability Statement
The datasets analyzed in the current study are available from the corresponding author on reasonable request.