Abstract
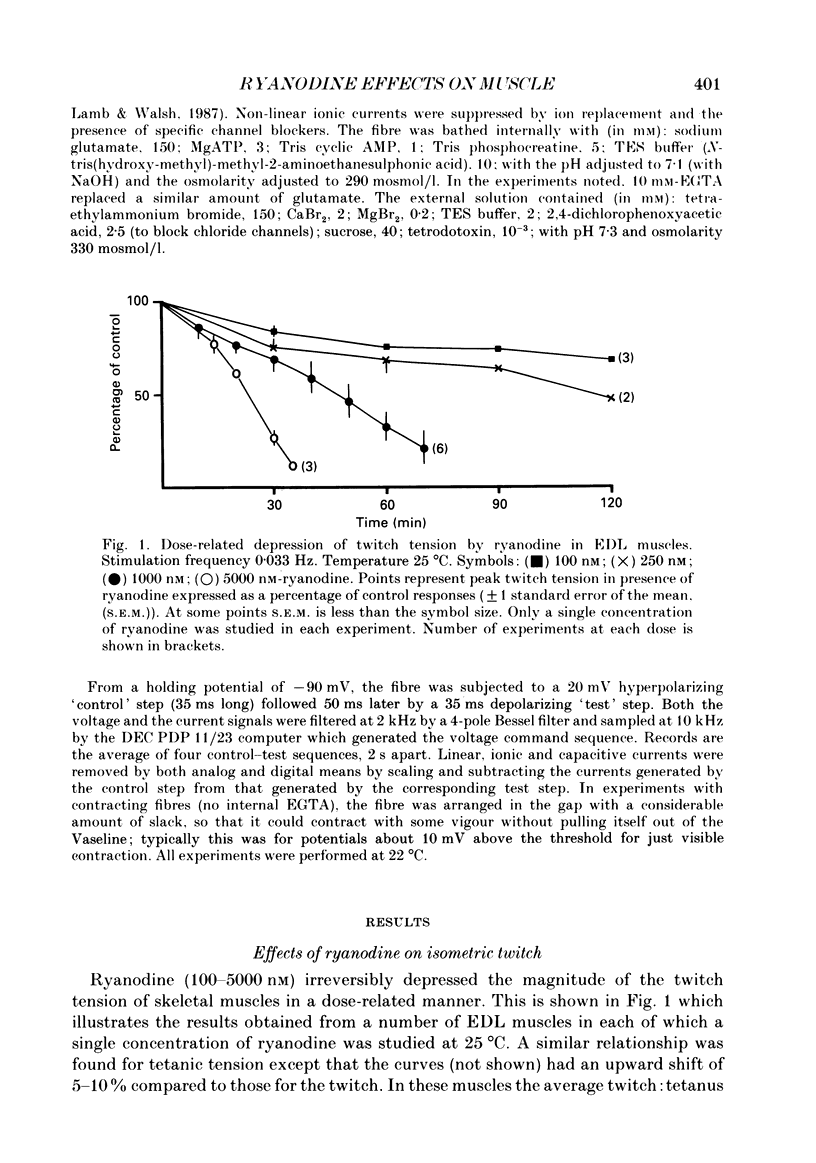
1. The action of ryanodine on force development of bundles dissected from rat extensor digitorum longus (EDL) and soleus muscles has been examined. 2. Ryanodine (100-5000 nM) irreversibly depressed twitch and tetanic tension of both muscle types in a dose-related manner. 3. At concentrations above 250 nM, ryanodine induced a slowly developing, dose-dependent contracture which could not be blocked by 5 mM-Co2+. Increasing the stimulation rate or decreasing the oxygenation of the preparation accelerated the rate of contracture development while the total removal of extracellular Ca2+ was required to prevent it. 4. Following the relaxation of the initial contracture (IC) in Ca2+-free solution, a second type of contracture (SC) could be induced by the readdition of Ca2+. This contracture differed from IC in that it was dependent on Ca2+ in the millimolar range and was prevented by 5 mM-Co2+. Both IC and SC were relaxed by perfusion with Ca2+-free, EGTA-containing solution. 5. Subcontracture doses of ryanodine (100 nM) markedly potentiated caffeine contractures of both muscle types. 6. Asymmetric charge movement in EDL fibres was recorded with the Vaseline-gap technique. The amount of charge moved near threshold was virtually unaffected by the presence of 10 microM-ryanodine over the time examined. 7. The results are consistent with the suggestion that ryanodine locks the calcium release channels of the sarcoplasmic reticulum (SR) in an open subconductance state with reduced conductance. It appears that lowering the external calcium concentration might still inactivate the release channels after they have been blocked open by ryanodine, possibly by an effect on the T-tubular voltage sensor.
Full text
PDF

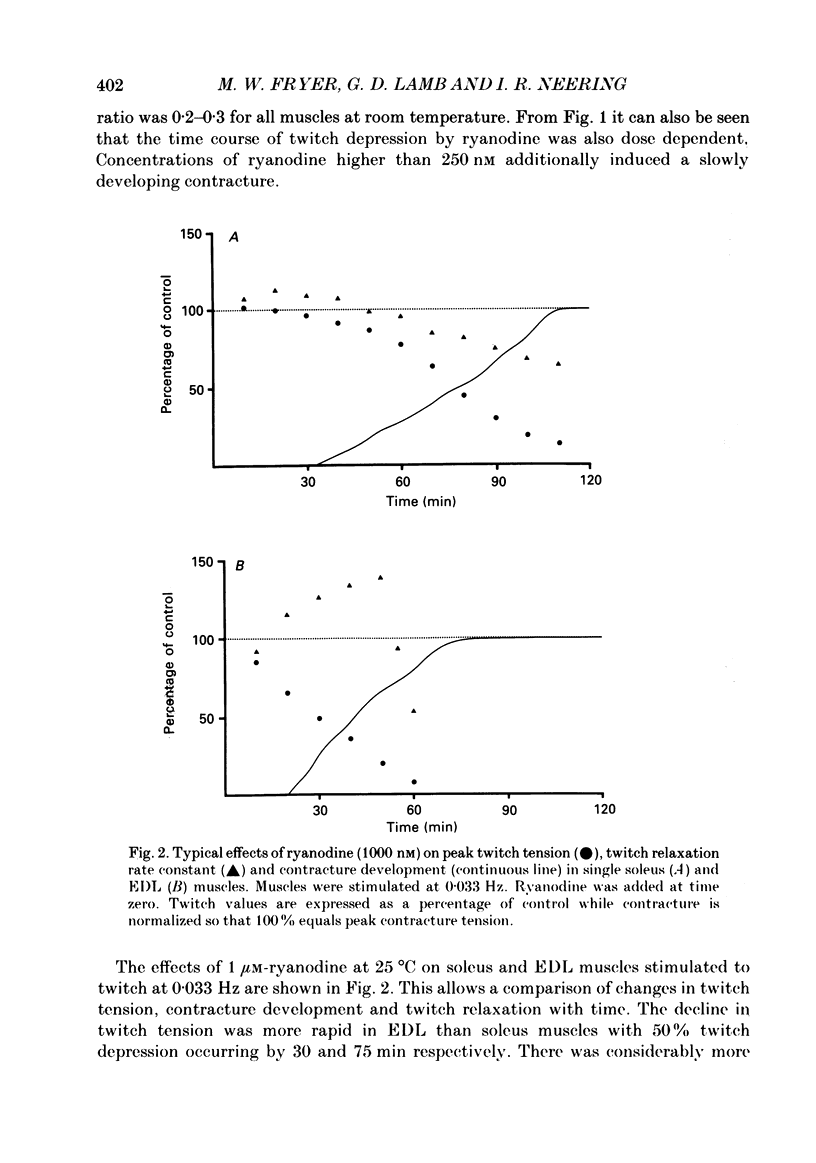

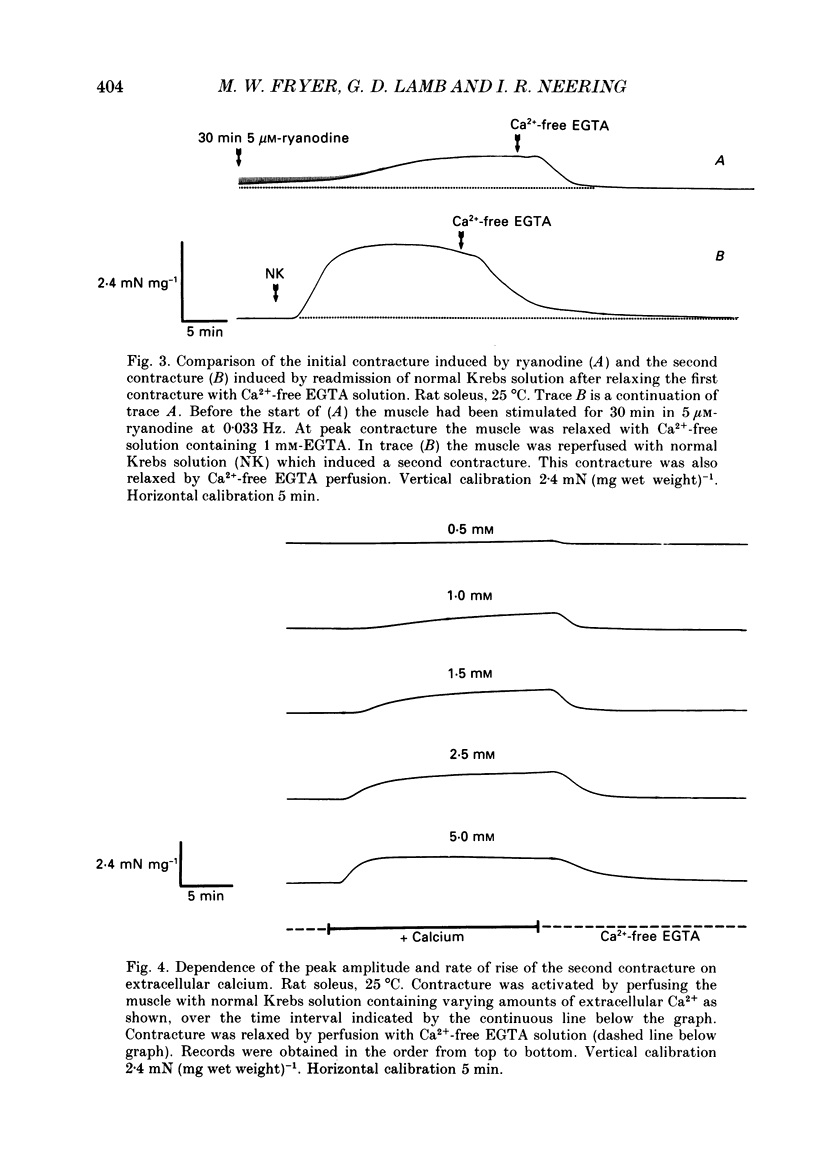
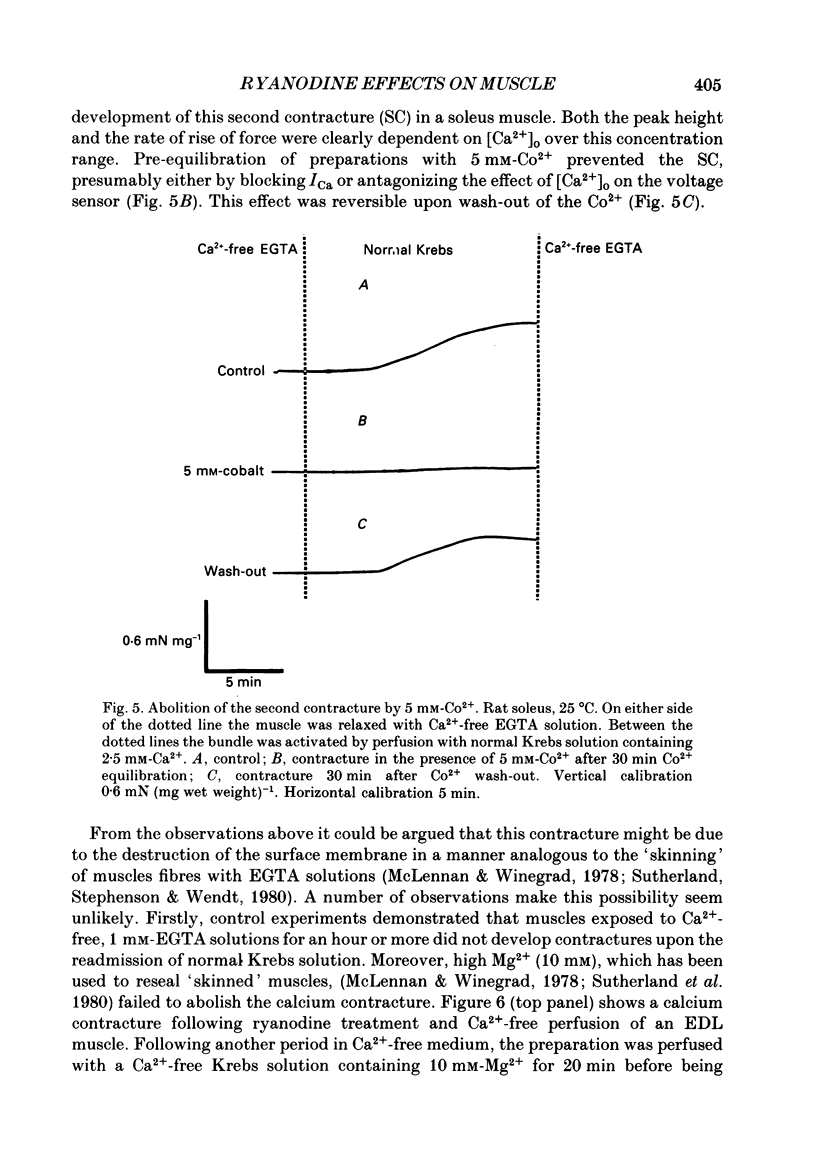






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong C. M., Bezanilla F. M., Horowicz P. Twitches in the presence of ethylene glycol bis( -aminoethyl ether)-N,N'-tetracetic acid. Biochim Biophys Acta. 1972 Jun 23;267(3):605–608. doi: 10.1016/0005-2728(72)90194-6. [DOI] [PubMed] [Google Scholar]
- BLUM J. J., CREESE R., JENDEN D. J., SCHOLES N. W. The mechanism of action of ryanodine on skeletal muscle. J Pharmacol Exp Ther. 1957 Dec;121(4):477–486. [PubMed] [Google Scholar]
- Bers D. M. Ryanodine and the calcium content of cardiac SR assessed by caffeine and rapid cooling contractures. Am J Physiol. 1987 Sep;253(3 Pt 1):C408–C415. doi: 10.1152/ajpcell.1987.253.3.C408. [DOI] [PubMed] [Google Scholar]
- Brum G., Fitts R., Pizarro G., Ríos E. Voltage sensors of the frog skeletal muscle membrane require calcium to function in excitation-contraction coupling. J Physiol. 1988 Apr;398:475–505. doi: 10.1113/jphysiol.1988.sp017053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brum G., Ríos E., Stéfani E. Effects of extracellular calcium on calcium movements of excitation-contraction coupling in frog skeletal muscle fibres. J Physiol. 1988 Apr;398:441–473. doi: 10.1113/jphysiol.1988.sp017052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G. A., Weiant E. A., Slocombe A. G., Roeder K. D. The Action of Ryanodine on the Contractile Process in Striated Muscle. Science. 1948 Sep 24;108(2804):330–332. doi: 10.1126/science.108.2804.330. [DOI] [PubMed] [Google Scholar]
- Graf F., Schatzmann H. J. Some effects of removal of external calcium on pig striated muscle. J Physiol. 1984 Apr;349:1–13. doi: 10.1113/jphysiol.1984.sp015138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajdu S. Mechanism of the Woodworth staircase phenomenon in heart and skeletal muscle. Am J Physiol. 1969 Jan;216(1):206–214. doi: 10.1152/ajplegacy.1969.216.1.206. [DOI] [PubMed] [Google Scholar]
- Hansford R. G., Lakatta E. G. Ryanodine releases calcium from sarcoplasmic reticulum in calcium-tolerant rat cardiac myocytes. J Physiol. 1987 Sep;390:453–467. doi: 10.1113/jphysiol.1987.sp016711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang K. S., Saida K., van Breemen C. Modulation of ryanodine-induced Ca2+ release in amphibian skeletal muscle. Biochem Biophys Res Commun. 1987 Feb 13;142(3):674–679. doi: 10.1016/0006-291x(87)91467-7. [DOI] [PubMed] [Google Scholar]
- Hwang K. S., van Breemen C. Ryanodine modulation of 45Ca efflux and tension in rabbit aortic smooth muscle. Pflugers Arch. 1987 Apr;408(4):343–350. doi: 10.1007/BF00581127. [DOI] [PubMed] [Google Scholar]
- Jenden D. J., Fairhurst A. S. The pharmacology of ryanodine. Pharmacol Rev. 1969 Mar;21(1):1–25. [PubMed] [Google Scholar]
- Jones L. R., Besch H. R., Jr, Sutko J. L., Willerson J. T. Ryanodine-induced stimulation of net Ca++ uptake by cardiac sarcoplasmic reticulum vesicles. J Pharmacol Exp Ther. 1979 Apr;209(1):48–55. [PubMed] [Google Scholar]
- Lamb G. D. Asymmetric charge movement in contracting muscle fibres in the rabbit. J Physiol. 1986 Jul;376:63–83. doi: 10.1113/jphysiol.1986.sp016142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb G. D., Walsh T. Calcium currents, charge movement and dihydropyridine binding in fast- and slow-twitch muscles of rat and rabbit. J Physiol. 1987 Dec;393:595–617. doi: 10.1113/jphysiol.1987.sp016843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüttgau H. C., Spiecker W. The effects of calcium deprivation upon mechanical and electrophysiological parameters in skeletal muscle fibres of the frog. J Physiol. 1979 Nov;296:411–429. doi: 10.1113/jphysiol.1979.sp013013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClellan G. B., Winegrad S. The regulation of the calcium sensitivity of the contractile system in mammalian cardiac muscle. J Gen Physiol. 1978 Dec;72(6):737–764. doi: 10.1085/jgp.72.6.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner G. Ryanodine activation and inhibition of the Ca2+ release channel of sarcoplasmic reticulum. J Biol Chem. 1986 May 15;261(14):6300–6306. [PubMed] [Google Scholar]
- Nelson T. E. Ryanodine: antithetical calcium channel effects in skeletal muscle sarcoplasmic reticulum. J Pharmacol Exp Ther. 1987 Jul;242(1):56–61. [PubMed] [Google Scholar]
- PROCITA L. The action of ryanodine on mammalian skeletal muscle in situ. J Pharmacol Exp Ther. 1956 Aug;117(4):363–373. [PubMed] [Google Scholar]
- Pessah I. N., Waterhouse A. L., Casida J. E. The calcium-ryanodine receptor complex of skeletal and cardiac muscle. Biochem Biophys Res Commun. 1985 Apr 16;128(1):449–456. doi: 10.1016/0006-291x(85)91699-7. [DOI] [PubMed] [Google Scholar]
- Rios E., Brum G. Involvement of dihydropyridine receptors in excitation-contraction coupling in skeletal muscle. Nature. 1987 Feb 19;325(6106):717–720. doi: 10.1038/325717a0. [DOI] [PubMed] [Google Scholar]
- Rousseau E., Smith J. S., Meissner G. Ryanodine modifies conductance and gating behavior of single Ca2+ release channel. Am J Physiol. 1987 Sep;253(3 Pt 1):C364–C368. doi: 10.1152/ajpcell.1987.253.3.C364. [DOI] [PubMed] [Google Scholar]
- SERAYDARIAN M. W., JENDEN D. J., ABBOTT B. C. The effect of ryanodine on relaxation of frog sartorius. J Pharmacol Exp Ther. 1962 Mar;135:374–381. [PubMed] [Google Scholar]
- Salviati G., Volpe P. Ca2+ release from sarcoplasmic reticulum of skinned fast- and slow-twitch muscle fibers. Am J Physiol. 1988 Mar;254(3 Pt 1):C459–C465. doi: 10.1152/ajpcell.1988.254.3.C459. [DOI] [PubMed] [Google Scholar]
- Schneider M. F., Chandler W. K. Voltage dependent charge movement of skeletal muscle: a possible step in excitation-contraction coupling. Nature. 1973 Mar 23;242(5395):244–246. doi: 10.1038/242244a0. [DOI] [PubMed] [Google Scholar]
- Su J. Y. Mechanisms of ryanodine-induced depression of caffeine-induced tension transients in skinned striated rabbit muscle fibers. Pflugers Arch. 1988 Apr;411(4):371–377. doi: 10.1007/BF00587715. [DOI] [PubMed] [Google Scholar]
- Sutko J. L., Ito K., Kenyon J. L. Ryanodine: a modifier of sarcoplasmic reticulum calcium release in striated muscle. Fed Proc. 1985 Dec;44(15):2984–2988. [PubMed] [Google Scholar]
- Sutko J. L., Willerson J. T., Templeton G. H., Jones L. R., Besch H. R., Jr Ryanodine: its alterations of cat papillary muscle contractile state and responsiveness to inotropic interventions and a suggested mechanism of action. J Pharmacol Exp Ther. 1979 Apr;209(1):37–47. [PubMed] [Google Scholar]
- Tanabe T., Beam K. G., Powell J. A., Numa S. Restoration of excitation-contraction coupling and slow calcium current in dysgenic muscle by dihydropyridine receptor complementary DNA. Nature. 1988 Nov 10;336(6195):134–139. doi: 10.1038/336134a0. [DOI] [PubMed] [Google Scholar]
- Wier W. G., Yue D. T., Marban E. Effects of ryanodine on intracellular Ca2+ transients in mammalian cardiac muscle. Fed Proc. 1985 Dec;44(15):2989–2993. [PubMed] [Google Scholar]


