Abstract
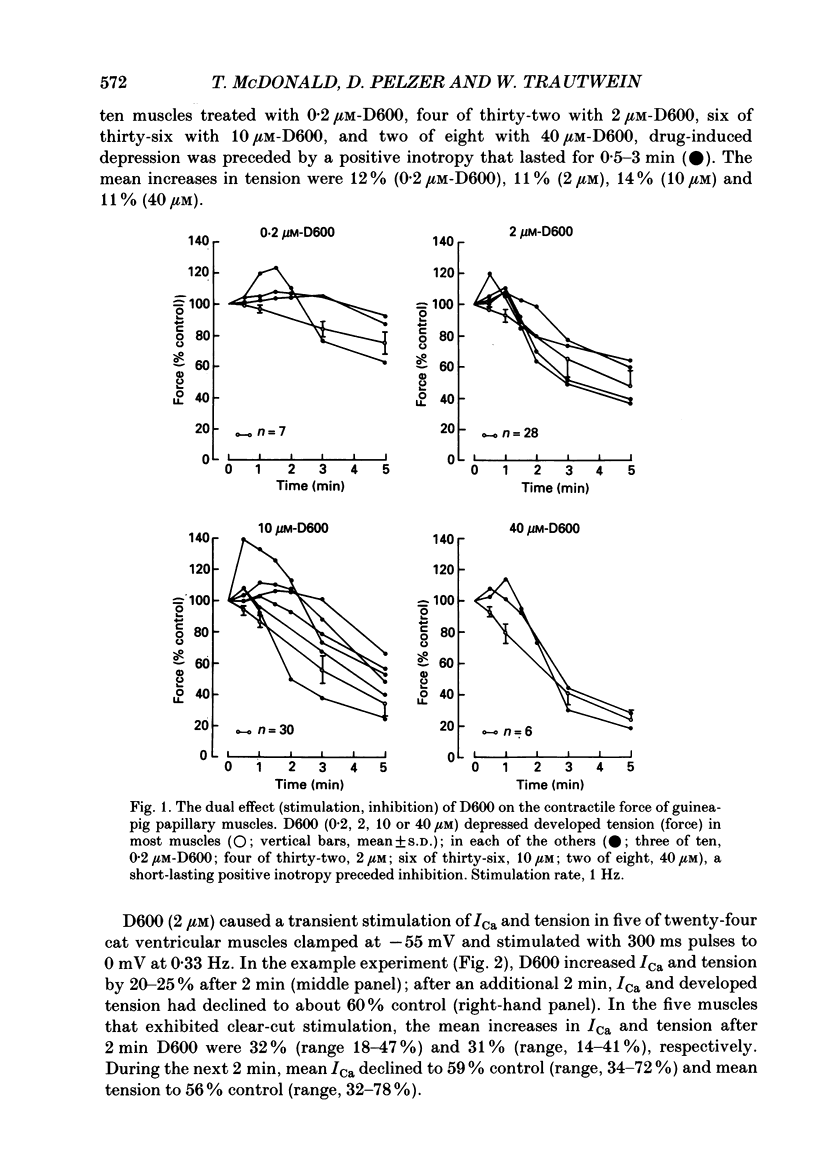
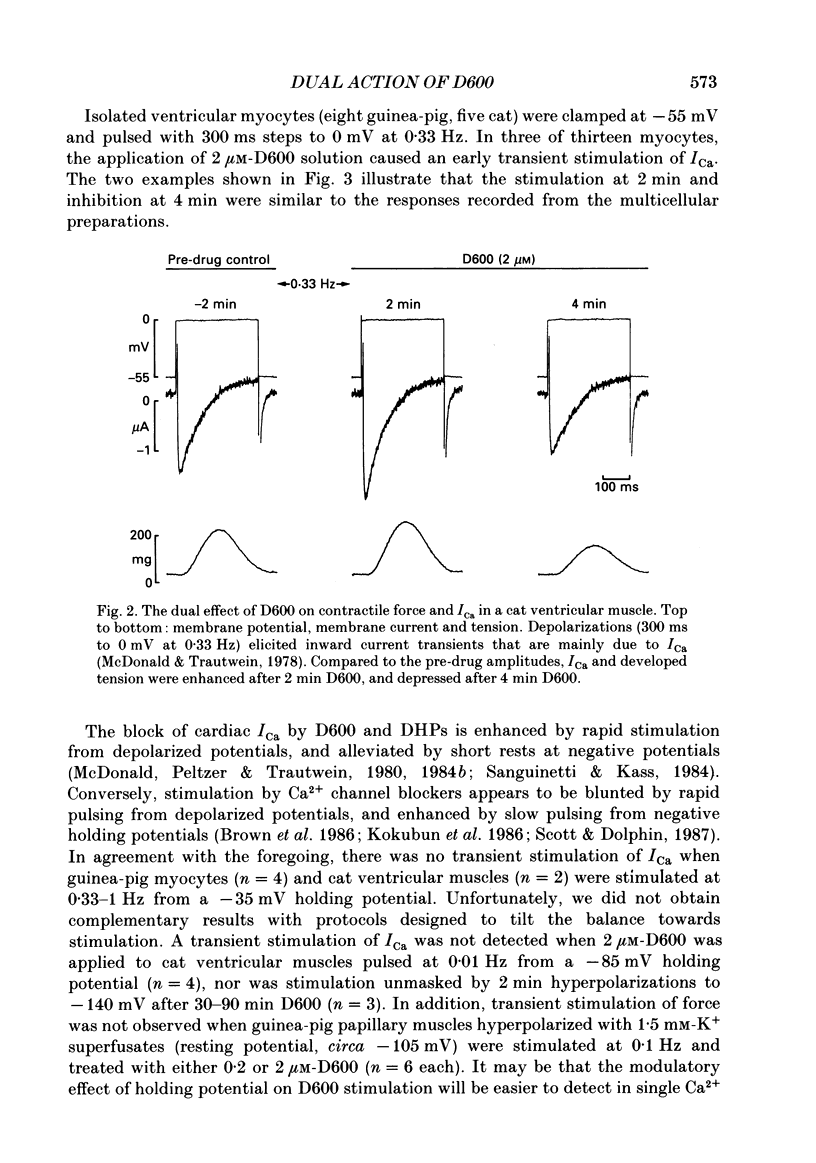
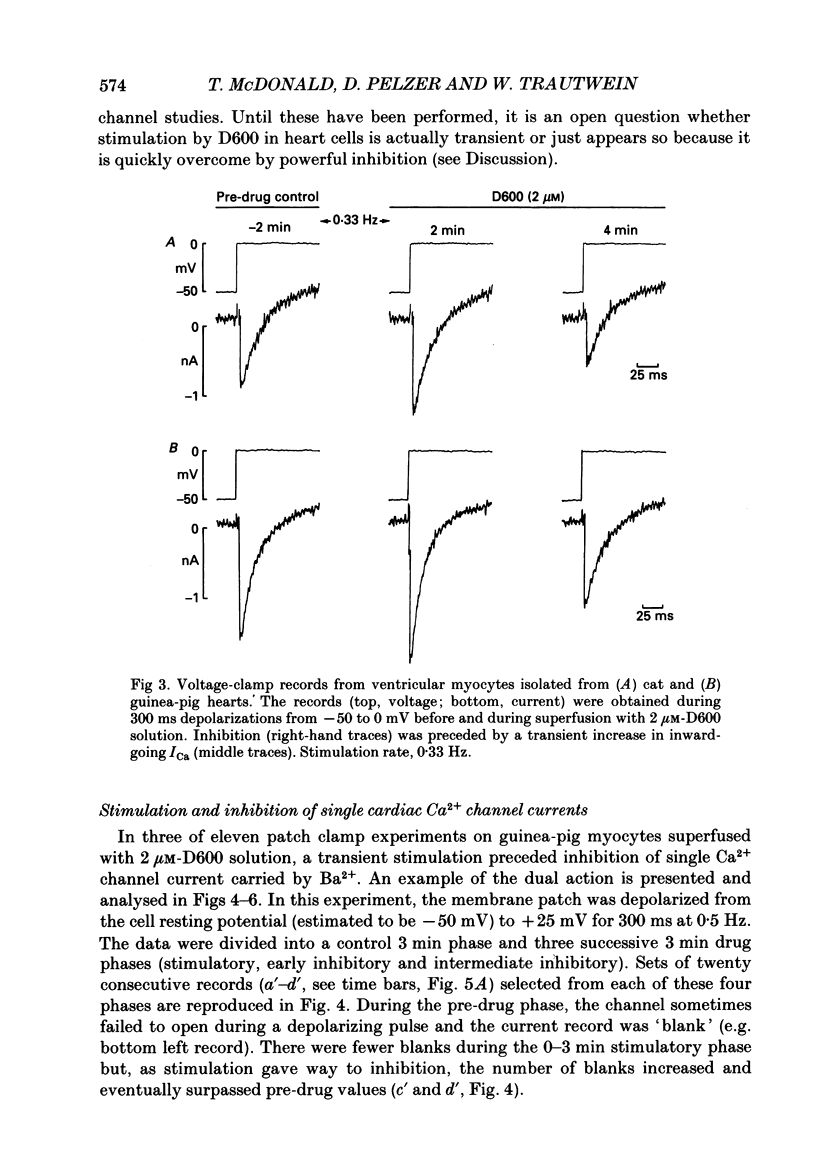
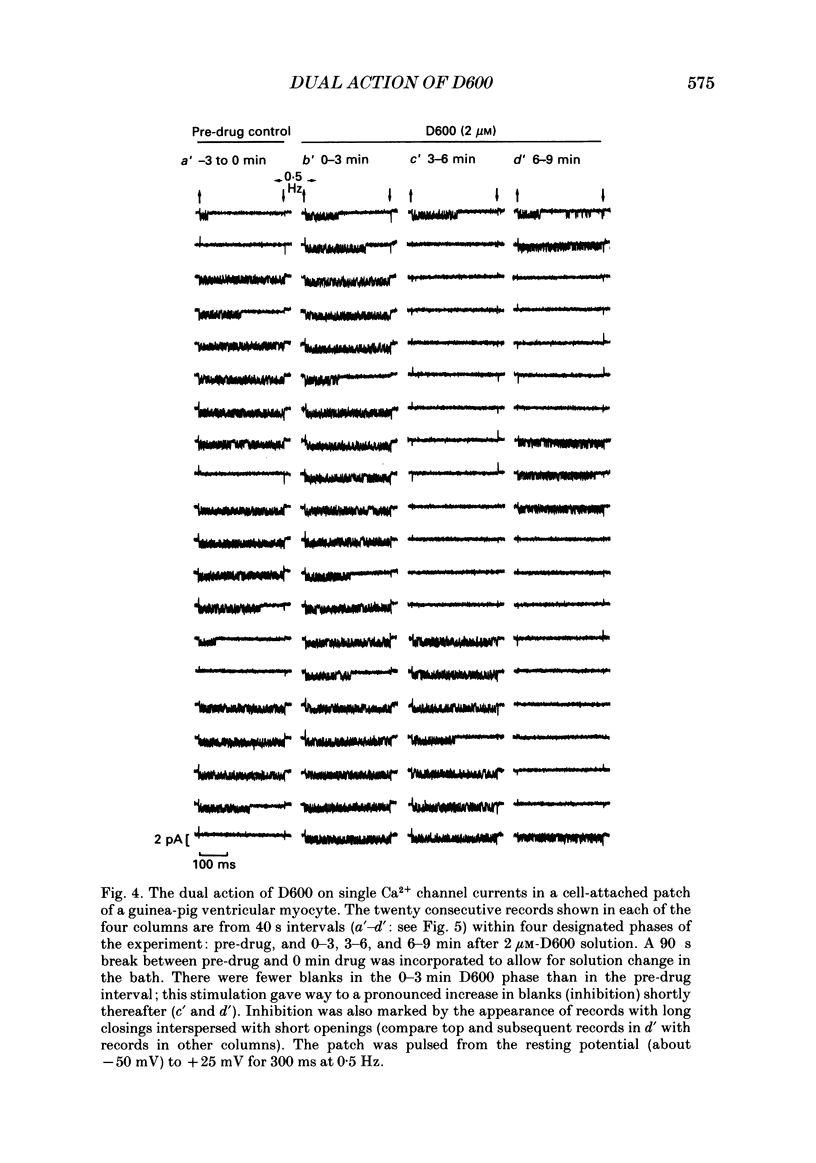
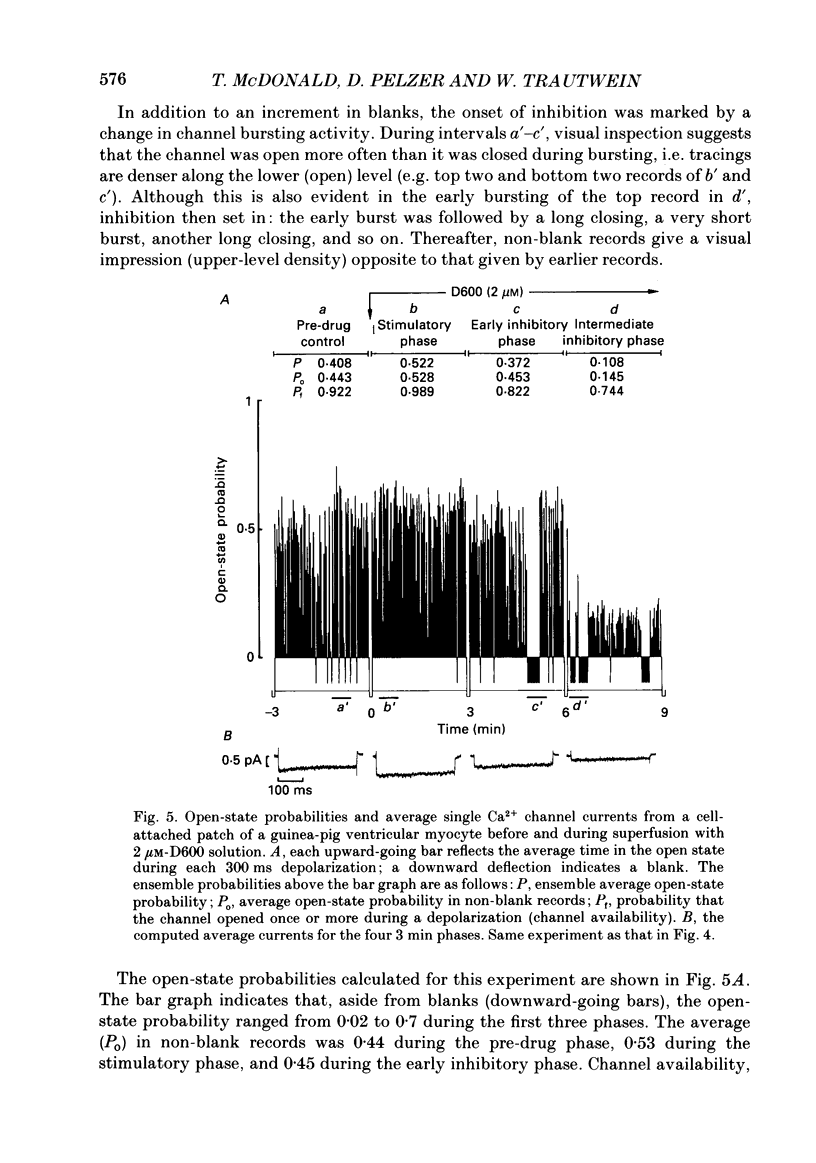
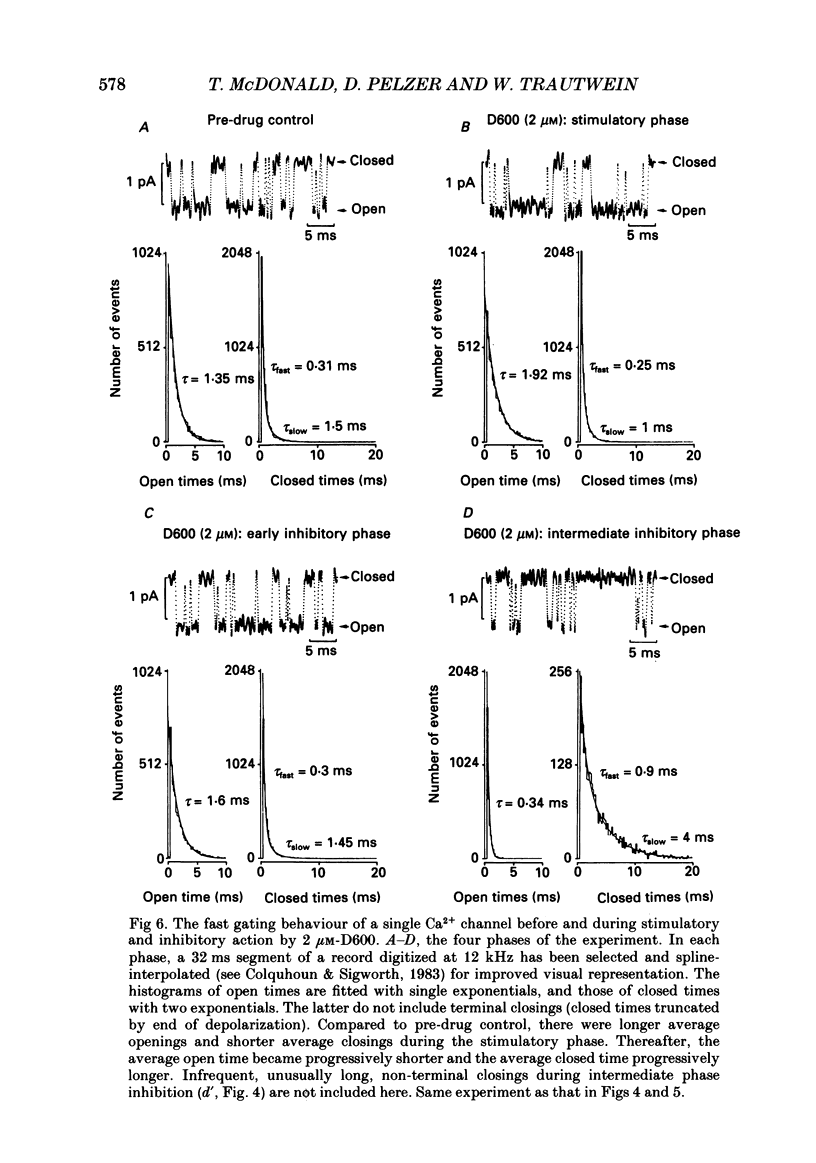
1. We examined the effects of D600 (0.2-40 microM, generally 2 microM) on the following (i) developed tension in guinea-pig papillary muscles, (ii) calcium current (Ica) and tension in cat ventricular muscle strands, (iii) Ica in guinea-pig and cat ventricular myocytes, (iv) single Ca2+ channel currents carried by Ba2+ in cell-attached membrane patches of guinea-pig ventricular myocytes, and (v) Ba2+ currents through dihydropyridine (DHP)-binding sites (skeletal muscle) reconstituted into single functional Ca2+ channels in lipid bilayers. 2. In 27 of 140 preparations studied, D600 elicited a transient stimulation that preceded marked inhibition. The stimulation was normally of short duration (less than 5 min) and moderate strength (less than 50% increase). 3. D600 had no effect on the unit conductance of single cardiac Ca2+ channels. Stimulation was characterized by a decrease in the number of records with no openings (blanks) and an increase in the open-state probability of non-blanks (longer open times, shorter closed times). Inhibition began with an increase in the number of blanks and later included a curtailment of open times and a prolongation of closed times. The net effect after 9 min D600 was a 75% reduction in average current amplitude. 4. A similar pattern of changes in channel open and closed times produced enhancement and then depression of time-averaged open-state probability in single reconstituted channels. 5. Single Ca2+ channel current that was stimulated by adrenaline was only slightly depressed after 2 microM-D600 for 30 min. It may be that channel phosphorylation or Gs-protein activation following beta-receptor stimulation reduces channel affinity for D600. 6. Short-lived binding of D600 to a single inhibitory site may enhance association/activation of Gs-protein and thereby cause transient up-regulation prior to increased drug occupancy and inhibition. Alternatively, there may be separate stimulatory and inhibitory sites. One aspect of inhibition, the increased frequency of blanks, is attributed to a stabilization of the inactivated state; the other aspect, changes in fast kinetics, seems to require a different explanation.
Full text
PDF




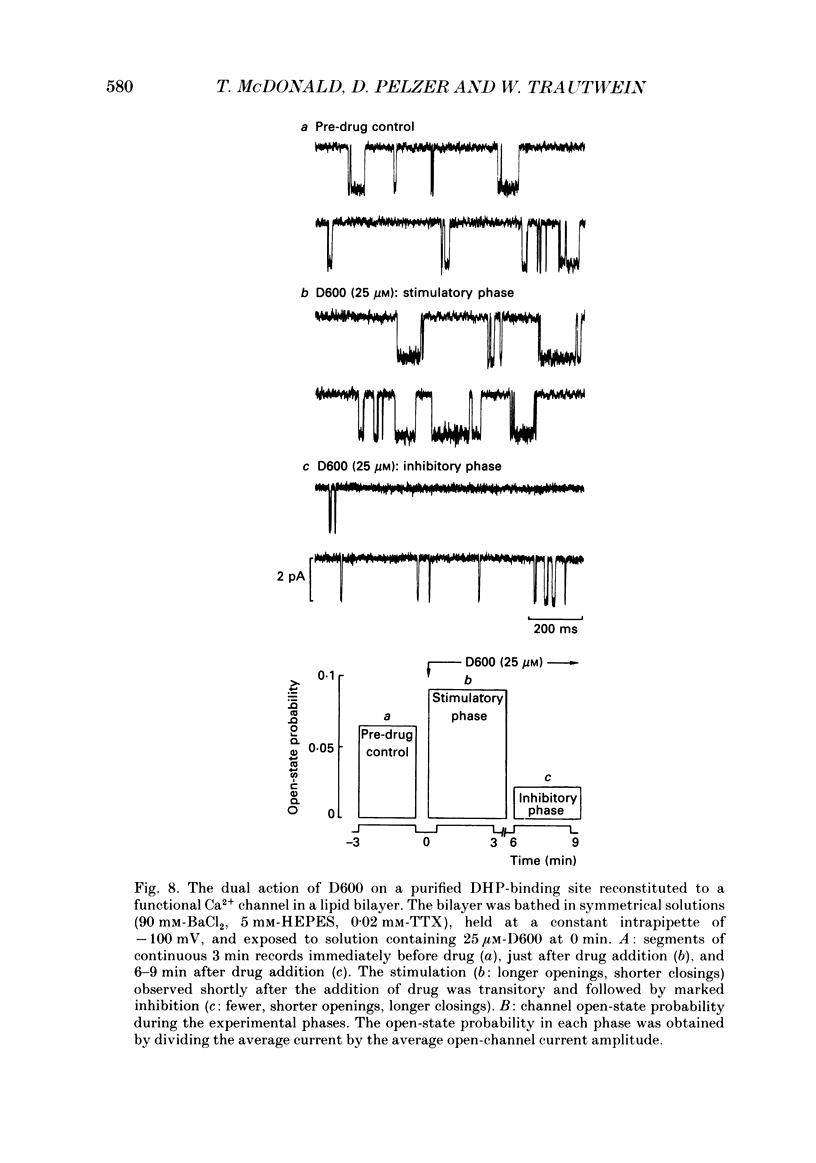






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berwe D., Gottschalk G., Lüttgau H. C. Effects of the calcium antagonist gallopamil (D600) upon excitation-contraction coupling in toe muscle fibres of the frog. J Physiol. 1987 Apr;385:693–707. doi: 10.1113/jphysiol.1987.sp016515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. M., Kunze D. L., Yatani A. Dual effects of dihydropyridines on whole cell and unitary calcium currents in single ventricular cells of guinea-pig. J Physiol. 1986 Oct;379:495–514. doi: 10.1113/jphysiol.1986.sp016266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalié A., Ochi R., Pelzer D., Trautwein W. Elementary currents through Ca2+ channels in guinea pig myocytes. Pflugers Arch. 1983 Sep;398(4):284–297. doi: 10.1007/BF00657238. [DOI] [PubMed] [Google Scholar]
- Cavalié A., Pelzer D., Trautwein W. Fast and slow gating behaviour of single calcium channels in cardiac cells. Relation to activation and inactivation of calcium-channel current. Pflugers Arch. 1986 Mar;406(3):241–258. doi: 10.1007/BF00640910. [DOI] [PubMed] [Google Scholar]
- Dörrscheidt-Käfer M. The action of D600 on frog skeletal muscle: facilitation of excitation-contraction coupling. Pflugers Arch. 1977 Jul 19;369(3):259–267. doi: 10.1007/BF00582193. [DOI] [PubMed] [Google Scholar]
- Eisenberg R. S., McCarthy R. T., Milton R. L. Paralysis of frog skeletal muscle fibres by the calcium antagonist D-600. J Physiol. 1983 Aug;341:495–505. doi: 10.1113/jphysiol.1983.sp014819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flockerzi V., Oeken H. J., Hofmann F., Pelzer D., Cavalié A., Trautwein W. Purified dihydropyridine-binding site from skeletal muscle t-tubules is a functional calcium channel. Nature. 1986 Sep 4;323(6083):66–68. doi: 10.1038/323066a0. [DOI] [PubMed] [Google Scholar]
- Frank G. B. Blockade of Ca2+ channels inhibits K+ contractures but not twitches in skeletal muscle. Can J Physiol Pharmacol. 1984 Apr;62(4):374–378. doi: 10.1139/y84-059. [DOI] [PubMed] [Google Scholar]
- Galizzi J. P., Fosset M., Lazdunski M. Properties of receptors for the Ca2+-channel blocker verapamil in transverse-tubule membranes of skeletal muscle. Stereospecificity, effect of Ca2+ and other inorganic cations, evidence for two categories of sites and effect of nucleoside triphosphates. Eur J Biochem. 1984 Oct 15;144(2):211–215. doi: 10.1111/j.1432-1033.1984.tb08451.x. [DOI] [PubMed] [Google Scholar]
- Hess P., Lansman J. B., Tsien R. W. Different modes of Ca channel gating behaviour favoured by dihydropyridine Ca agonists and antagonists. Nature. 1984 Oct 11;311(5986):538–544. doi: 10.1038/311538a0. [DOI] [PubMed] [Google Scholar]
- Iijima T., Yanagisawa T., Taira N. Increase in the slow inward current by intracellularly applied nifedipine and nicardipine in single ventricular cells of the guinea-pig heart. J Mol Cell Cardiol. 1984 Dec;16(12):1173–1177. doi: 10.1016/s0022-2828(84)80043-7. [DOI] [PubMed] [Google Scholar]
- Kohlhardt M., Bauer B., Krause H., Fleckenstein A. Differentiation of the transmembrane Na and Ca channels in mammalian cardiac fibres by the use of specific inhibitors. Pflugers Arch. 1972;335(4):309–322. doi: 10.1007/BF00586221. [DOI] [PubMed] [Google Scholar]
- Kokubun S., Prod'hom B., Becker C., Porzig H., Reuter H. Studies on Ca channels in intact cardiac cells: voltage-dependent effects and cooperative interactions of dihydropyridine enantiomers. Mol Pharmacol. 1986 Dec;30(6):571–584. [PubMed] [Google Scholar]
- Lee K. S., Tsien R. W. Mechanism of calcium channel blockade by verapamil, D600, diltiazem and nitrendipine in single dialysed heart cells. Nature. 1983 Apr 28;302(5911):790–794. doi: 10.1038/302790a0. [DOI] [PubMed] [Google Scholar]
- Marwaha J., Treffers R. C. Actions of a calium antagonist, the D-600, on electrical and mechanical properties of frog skeletal muscle. Prog Neuropsychopharmacol. 1980;4(2):145–152. doi: 10.1016/0364-7722(80)90031-4. [DOI] [PubMed] [Google Scholar]
- McDonald T. F., Cavalié A., Trautwein W., Pelzer D. Voltage-dependent properties of macroscopic and elementary calcium channel currents in guinea pig ventricular myocytes. Pflugers Arch. 1986 May;406(5):437–448. doi: 10.1007/BF00583365. [DOI] [PubMed] [Google Scholar]
- McDonald T. F., Pelzer D., Trautwein W. Cat ventricular muscle treated with D600: characteristics of calcium channel block and unblock. J Physiol. 1984 Jul;352:217–241. doi: 10.1113/jphysiol.1984.sp015288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald T. F., Pelzer D., Trautwein W. Cat ventricular muscle treated with D600: effects on calcium and potassium currents. J Physiol. 1984 Jul;352:203–216. doi: 10.1113/jphysiol.1984.sp015287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald T. F., Pelzer D., Trautwein W. On the mechanism of slow calcium channel block in heart. Pflugers Arch. 1980 May;385(2):175–179. doi: 10.1007/BF00588699. [DOI] [PubMed] [Google Scholar]
- McDonald T. F., Trautwein W. Membrane currents in cat myocardium: separation of inward and outward components. J Physiol. 1978 Jan;274:193–216. doi: 10.1113/jphysiol.1978.sp012143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrath H., Eick R. E., McDonald T. F., Trautwein W. On the mechanism underlying the action of D-600 on slow inward current and tension in mammalian myocardium. Circ Res. 1977 Apr;40(4):408–414. doi: 10.1161/01.res.40.4.408. [DOI] [PubMed] [Google Scholar]
- Osterrieder W., Brum G., Hescheler J., Trautwein W., Flockerzi V., Hofmann F. Injection of subunits of cyclic AMP-dependent protein kinase into cardiac myocytes modulates Ca2+ current. Nature. 1982 Aug 5;298(5874):576–578. doi: 10.1038/298576a0. [DOI] [PubMed] [Google Scholar]
- Palade P. T., Almers W. Slow calcium and potassium currents in frog skeletal muscle: their relationship and pharmacologic properties. Pflugers Arch. 1985 Sep;405(2):91–101. doi: 10.1007/BF00584528. [DOI] [PubMed] [Google Scholar]
- Pelzer D., Trautwein W., McDonald T. F. Calcium channel block and recovery from block in mammalian ventricular muscle treated with organic channel inhibitors. Pflugers Arch. 1982 Aug;394(2):97–105. doi: 10.1007/BF00582909. [DOI] [PubMed] [Google Scholar]
- Sanguinetti M. C., Kass R. S. Voltage-dependent block of calcium channel current in the calf cardiac Purkinje fiber by dihydropyridine calcium channel antagonists. Circ Res. 1984 Sep;55(3):336–348. doi: 10.1161/01.res.55.3.336. [DOI] [PubMed] [Google Scholar]
- Schneider M. F., Chandler W. K. Voltage dependent charge movement of skeletal muscle: a possible step in excitation-contraction coupling. Nature. 1973 Mar 23;242(5395):244–246. doi: 10.1038/242244a0. [DOI] [PubMed] [Google Scholar]
- Scott R. H., Dolphin A. C. Activation of a G protein promotes agonist responses to calcium channel ligands. Nature. 1987 Dec 24;330(6150):760–762. doi: 10.1038/330760a0. [DOI] [PubMed] [Google Scholar]
- Tsien R. W., Bean B. P., Hess P., Lansman J. B., Nilius B., Nowycky M. C. Mechanisms of calcium channel modulation by beta-adrenergic agents and dihydropyridine calcium agonists. J Mol Cell Cardiol. 1986 Jul;18(7):691–710. doi: 10.1016/s0022-2828(86)80941-5. [DOI] [PubMed] [Google Scholar]
- Uehara A., Hume J. R. Interactions of organic calcium channel antagonists with calcium channels in single frog atrial cells. J Gen Physiol. 1985 May;85(5):621–647. doi: 10.1085/jgp.85.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Rautaharju P. M., McDonald T. F. Ventricular action potentials, ventricular extracellular potentials, and the ECG of guinea pig. Circ Res. 1985 Sep;57(3):362–373. doi: 10.1161/01.res.57.3.362. [DOI] [PubMed] [Google Scholar]
- Yatani A., Codina J., Imoto Y., Reeves J. P., Birnbaumer L., Brown A. M. A G protein directly regulates mammalian cardiac calcium channels. Science. 1987 Nov 27;238(4831):1288–1292. doi: 10.1126/science.2446390. [DOI] [PubMed] [Google Scholar]


