Abstract
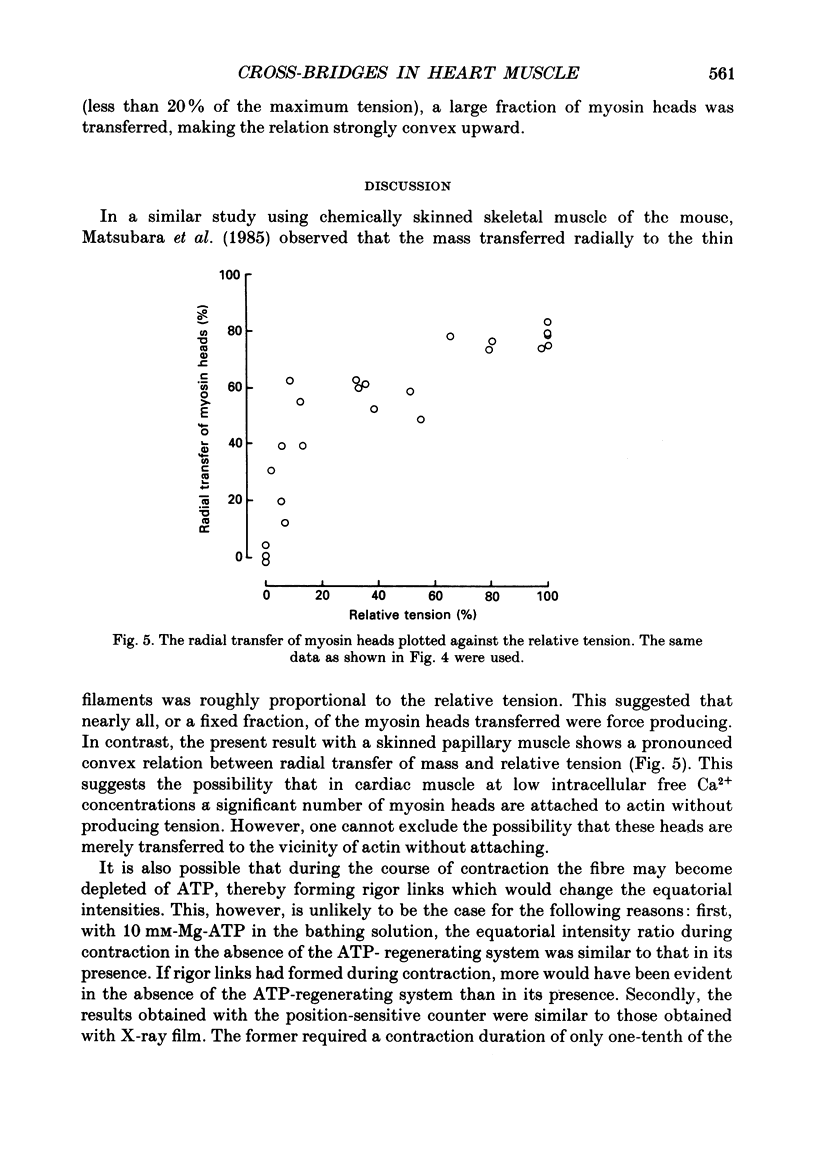
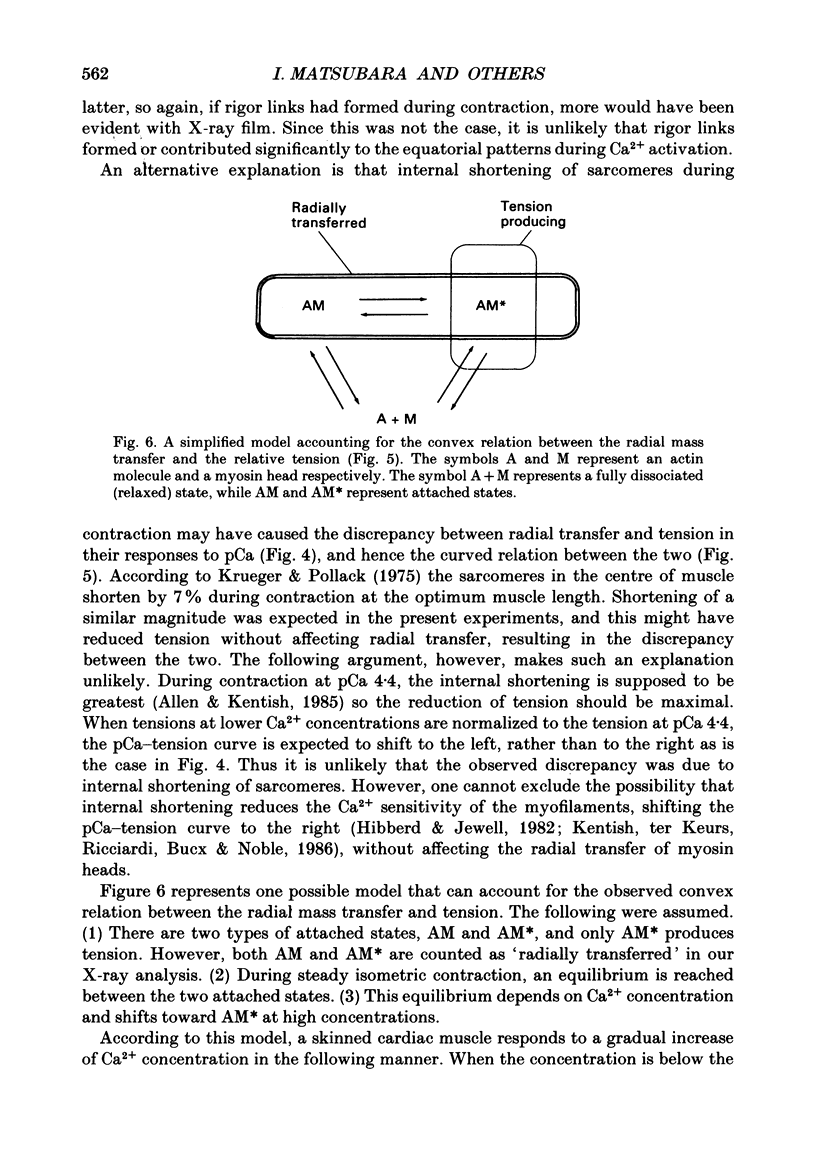
1. By applying the X-ray diffraction method to chemically skinned papillary muscles of the rat, the transfer of myosin heads from the thick to the thin filaments was studied as a function of Ca2+ concentration. 2. No significant transfer of the heads occurred when the Ca2+ concentration was below the threshold of contraction (pCa 6.2). 3. During the maximum isometric contraction at pCa 4.4, 80% of the myosin heads were transferred to the thin filament. 4. When the muscle was activated isometrically at low Ca2+ concentrations (pCa 6.2-5.8), where the average tension was less than 20% of the maximum, a disproportionately large number of myosin heads were transferred to the thin filament. 5. It was concluded that a significant fraction of the heads transferred at the low Ca2+ concentrations does not produce tension.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Kentish J. C. The cellular basis of the length-tension relation in cardiac muscle. J Mol Cell Cardiol. 1985 Sep;17(9):821–840. doi: 10.1016/s0022-2828(85)80097-3. [DOI] [PubMed] [Google Scholar]
- Brenner B., Schoenberg M., Chalovich J. M., Greene L. E., Eisenberg E. Evidence for cross-bridge attachment in relaxed muscle at low ionic strength. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7288–7291. doi: 10.1073/pnas.79.23.7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLIOTT G. F., WORTHINGTON C. R. A SMALL-ANGLE OPTICALLY FOCUSING X-RAY DIFFRACTION CAMERA IN BIOLOGICAL RESEARCH. I. J Ultrastruct Res. 1963 Aug;49:166–170. doi: 10.1016/s0022-5320(63)80044-1. [DOI] [PubMed] [Google Scholar]
- Eisenberg E., Hill T. L. Muscle contraction and free energy transduction in biological systems. Science. 1985 Mar 1;227(4690):999–1006. doi: 10.1126/science.3156404. [DOI] [PubMed] [Google Scholar]
- Harafuji H., Ogawa Y. Re-examination of the apparent binding constant of ethylene glycol bis(beta-aminoethyl ether)-N,N,N',N'-tetraacetic acid with calcium around neutral pH. J Biochem. 1980 May;87(5):1305–1312. doi: 10.1093/oxfordjournals.jbchem.a132868. [DOI] [PubMed] [Google Scholar]
- Haselgrove J. C., Huxley H. E. X-ray evidence for radial cross-bridge movement and for the sliding filament model in actively contracting skeletal muscle. J Mol Biol. 1973 Jul 15;77(4):549–568. doi: 10.1016/0022-2836(73)90222-2. [DOI] [PubMed] [Google Scholar]
- Herzig J. W., Rüegg J. C. Investigations on glycerinated cardiac muscle fibres in relation to the problem of regulation of cardiac contractility--effects of Ca++ and c-AMP. Basic Res Cardiol. 1980 Jan-Feb;75(1):26–33. doi: 10.1007/BF02001390. [DOI] [PubMed] [Google Scholar]
- Hibberd M. G., Jewell B. R. Calcium- and length-dependent force production in rat ventricular muscle. J Physiol. 1982 Aug;329:527–540. doi: 10.1113/jphysiol.1982.sp014317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley H. E. Structural difference between resting and rigor muscle; evidence from intensity changes in the lowangle equatorial x-ray diagram. J Mol Biol. 1968 Nov 14;37(3):507–520. doi: 10.1016/0022-2836(68)90118-6. [DOI] [PubMed] [Google Scholar]
- Katz A. M. Contractile proteins of the heart. Physiol Rev. 1970 Jan;50(1):63–158. doi: 10.1152/physrev.1970.50.1.63. [DOI] [PubMed] [Google Scholar]
- Kentish J. C., ter Keurs H. E., Ricciardi L., Bucx J. J., Noble M. I. Comparison between the sarcomere length-force relations of intact and skinned trabeculae from rat right ventricle. Influence of calcium concentrations on these relations. Circ Res. 1986 Jun;58(6):755–768. doi: 10.1161/01.res.58.6.755. [DOI] [PubMed] [Google Scholar]
- Khan M. M., Martell A. E. Thermodynamic quantities associated with the interaction of adenosine triphosphate with metal ions. J Am Chem Soc. 1966 Feb 20;88(4):668–671. doi: 10.1021/ja00956a008. [DOI] [PubMed] [Google Scholar]
- Krueger J. W., Pollack G. H. Myocardial sarcomere dynamics during isometric contraction. J Physiol. 1975 Oct;251(3):627–643. doi: 10.1113/jphysiol.1975.sp011112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara I., Millman B. M. X-ray diffraction patterns from mammalian heart muscle. J Mol Biol. 1974 Feb 5;82(4):527–536. doi: 10.1016/0022-2836(74)90246-0. [DOI] [PubMed] [Google Scholar]
- Matsubara I., Suga H., Yagi N. An X-ray diffraction study of the cross-circulated canine heart. J Physiol. 1977 Sep;270(2):311–320. doi: 10.1113/jphysiol.1977.sp011954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara I., Umazume Y., Yagi N. Lateral filamentary spacing in chemically skinned murine muscles during contraction. J Physiol. 1985 Mar;360:135–148. doi: 10.1113/jphysiol.1985.sp015608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara I. X-ray diffraction studies of the heart. Annu Rev Biophys Bioeng. 1980;9:81–105. doi: 10.1146/annurev.bb.09.060180.000501. [DOI] [PubMed] [Google Scholar]
- Matsubara I., Yagi N. A time-resolved X-ray diffraction study of muscle during twitch. J Physiol. 1978 May;278:297–307. doi: 10.1113/jphysiol.1978.sp012305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara I., Yagi N., Hashizume H. Use of an X-ray television for diffraction of the frog striated muscle. Nature. 1975 Jun 26;255(5511):728–729. doi: 10.1038/255728a0. [DOI] [PubMed] [Google Scholar]
- Moisescu D. G. Kinetics of reaction in calcium-activated skinned muscle fibres. Nature. 1976 Aug 12;262(5569):610–613. doi: 10.1038/262610a0. [DOI] [PubMed] [Google Scholar]
- Rome E. Light and X-ray diffraction studies of the filament lattice of glycerol-extracted rabbit psoas muscle. J Mol Biol. 1967 Aug 14;27(3):591–602. doi: 10.1016/0022-2836(67)90061-7. [DOI] [PubMed] [Google Scholar]
- Thomas D. D., Cooke R. Orientation of spin-labeled myosin heads in glycerinated muscle fibers. Biophys J. 1980 Dec;32(3):891–906. doi: 10.1016/S0006-3495(80)85024-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue D. T., Marban E., Wier W. G. Relationship between force and intracellular [Ca2+] in tetanized mammalian heart muscle. J Gen Physiol. 1986 Feb;87(2):223–242. doi: 10.1085/jgp.87.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]


