Abstract
Prior work has highlighted the role of genetic variation within the repetitive sequence in the transcriptional control region of the serotonin (5-HT) transporter gene (5-HTT, SLC6A4) in modulating amygdala and prefrontal activation to negative emotional stimuli. However, these studies have not explicitly tested the assumption that the control condition (neutral baseline) does not itself produce changes in activation as a function of 5-HTT genotype. Using a fixation baseline condition, we show that variation in 5-HTT genotype is associated with differential activation to negative, positive, and neutral stimuli in limbic, striatal, and cortical regions. We replicate earlier reports of increased amygdala activation to negative, relative to neutral, stimuli, but then show that these differences are driven by decreased activation to neutral stimuli, rather than increased activation to negative stimuli, in carriers of the 5-HTT short allele. Using high-resolution structural images and automated processes to test for brain volume and gray matter density, we further report significant differences, as a function of 5-HTT genotype, in frontal cortical regions, anterior cingulate, and cerebellum. These functional and structural differences suggest a much broader role for 5-HT transport efficiency in brain processes than previously thought. 5-HTT genotype affects neural systems controlling affective, cognitive, and motor processes.
Keywords: amygdala, polymorphism, 5-HTT
Individual differences in anxiety-related personality traits have been associated with polymorphisms within specific genes. For example, the short, 14-unit variant of a repetitive sequence in the transcriptional control region of the 5-HT transporter (5-HTT, SLC6A4) has been associated with neuroticism and harm avoidance (1, 2). A series of recent genomic imaging studies has begun to explore the hypothesis that the association between the 5-HTT polymorphism and these personality traits is mediated by neural systems processing negative affect.
The first study was conducted by Hariri and colleagues (3), and later extended by that group (4, 5). These investigators used functional MRI (fMRI) to show an association between the 5-HTT short allele and amygdala activation in an emotional face-matching task, compared with a visuospatial matching task. Furmark et al. (6) scanned social phobic patients by using [H2 15O] positron-emission tomography (PET) to report greater amygdala activation in short allele carriers during a public, compared with a private, speaking task. Heinz et al. (7) used fMRI to report a correlation between amygdala and ventromedial prefrontal cortex as a function of the 5-HTT short allele, which was specific to presentation of negative (but not positive), relative to neutral, images.
Thus, across several independent studies, a picture has emerged that suggests involvement of the 5-HTT short allele in enhancing brain reactivity to negative stimuli that may serve as a genetic susceptibility mechanism for depression (5), consistent with its role in negative affective traits. However, several important questions remain. First, it is unknown whether 5-HTT genotype may not modulate brain activation to neutral stimuli. None of the prior studies compared the neutral condition against another lower-level baseline condition. It is therefore unknown whether some of the reported effects are not driven by decreased activation to neutral, rather than increased activation to negative, stimuli in carriers of the short allele, relative to homozygous long allele carriers. This possibility would have significant consequences because it would suggest that 5-HTT genotype alters neural transmission beyond the processing of emotional stimuli, and may therefore play a broader role in cognition. The focus of prior studies on negative affect also limits what is known about functional and structural features affected by the 5-HTT polymorphism, because analyses were confined to a priori regions of interest. Therefore, a second question is whether 5-HTT genotype affects neural activation or structural features outside the amygdala and regions functionally connected to it (5, 7).
The current fMRI study therefore addressed the degree to which 5-HTT genotype affects neural activation to negative, positive, and neutral stimuli, using both a neutral and a fixation baseline condition. We used a variant of an attentional interference task first used in the scanner by Pardo et al. (8), the Stroop. This task can be sensitive to individual differences in personality and mood (9, 10), and activates the cognitive division (11) and the affective division of the anterior cingulate cortex (12), and amygdala (13), depending on whether the stimuli are neutral or affectively valenced. The anterior cingulate cortex plays an integral role in both affective and (nonaffective) cognitive processes and is heavily interconnected with a number of cortical and subcortical regions (14-21). In addition to functional analyses, we used an automated process to identify regions where volume or gray matter density varied as a function of 5-HTT genotype.
Methods
Subjects. Forty-one right-handed adults (19 males) with no reported history of psychiatric or neurological illness participated in this study. Subjects were divided into two groups, based on 5-HTT genotype. One consisted of carriers of either one (n = 16; 9 males) or two (n = 12; 4 males) copies of the 5-HTT short variant (S group), the other consisted of non-carriers (L group), i.e., individuals who are homozygous for the 5-HTT long variant (n = 13; 6 males). This grouping was made a priori, based on the observation that 5-HT uptake was ≈2-fold higher in human lymphoblastoid cells homozygous for the 5-HTT long variant, compared with cells that carried either one or two copies of the 5-HTT short variant, which had comparable 5-HT uptake (22). This functional dichotomy was subsequently confirmed by several different approaches at both the pre- and postsynaptic level (for review, see refs. 23 and 24). There were no significant differences between the S and L groups in age, self-reported mood state, or trait neuroticism, nor were there significant differences in the frequency of either gender or ethnicity. All subjects gave informed consent and all procedures were approved by the Institutional Review Boards of Stony Brook University and Yale University.
Behavioral Task Procedure. Subjects viewed four 18-second blocks of negative, neutral, or positive words from a standardized stimulus set (25) and fixation crosses (order counterbalanced across subjects) with six stimuli per block (1.5-s duration, 1.5 s inter-trial interval), and no repetitions of word stimuli. Each word was displayed on a computer screen in blue, red, or green, and subjects were instructed to press a button corresponding to the word color as quickly and accurately as possible, by using their right hand. There was no motor response to fixation stimuli.
fMRI. Whole-brain imaging data were acquired on a 3T Siemens (Iselin, NJ) Trio Scanner. Anatomical in-plane scans were conducted by using a T1 flash sequence, with 24 5-mm oblique slices aligned to the anterior commissure-posterior commissure (AC-PC) line [field of view (FOV) = 220 × 220; repetition time (TR) = 300; echo time (TE) = 2.47; flip angle = 60°; matrix = 256 × 256). For functional images, 24 5-mm oblique slices with an in-plane resolution of 3.44 mm, parallel to the AC-PC line, were collected by using an echoplanar imaging (EPI) scan sequence (FOV = 220 × 220, TR = 1,500, TE = 30, flip angle = 80°, matrix = 64 × 64). Using spm2 (Wellcome Department of Imaging Neuroscience), functional data were preprocessed, normalized to the gray matter template, and spatially smoothed with an 8-mm full-width, half-maximum isotropic Gaussian filter. Contrast images were created for each subject for neutral, negative, or positive stimuli versus a fixation baseline condition, and for negative and positive stimuli versus a neutral baseline condition. Fixed-effects models (26) were used at the individual subject level of analysis, and random-effects models (27) were used for group-level analyses. Activation differences based on 5-HTT genotype were calculated with a two-sample t test (S group versus L group). Given the prior literature, the amygdala was designated as an a priori region of interest, and activation within this region was considered significant at P < 0.05, false-discovery rate (FDR) corrected with an extent threshold of 10 voxels. Unless stated otherwise, for whole-brain analyses, we used a significance threshold of P = 0.05, uncorrected, with an extent threshold of 40 voxels (see Study Limitations and Statistical Considerations for more detail regarding this procedure). This combination of an activation and an extent threshold appropriately protects against false positives and corresponds to a per-pixel false positive correction of P = 0.0008, extrapolated from Forman et al. (28).
Voxel-Based Morphometry (VBM). High-resolution images for VBM analysis were acquired with a sagittal 3D MPRAGE sequence (T1 = 1,100, TR = 2530, TE = 3.66, flip angle = 7°, matrix = 256 × 256). VBM is an objective structural analysis that compares gray matter volume and density between groups (29, 30). Each subject's structural images were preprocessed according to an optimized VBM protocol (31). We then created a study-specific T1 brain template for spatial normalization from all subjects and study-specific probability maps to optimize the segmentation of each subject's image. The customized T1 template, gray matter, white matter, and cerebrospinal fluid images were used for the optimized VBM procedure. The spatially normalized segments of the each subject's gray matter images were modulated for volume analysis. The unmodulated images were used for density analysis. Both unmodulated and modulated images were smoothed with a filter of 10 mm full-with at half-maximum to increase signal-to-noise ratio. The ummodulated/modulated, smoothed images were analyzed with an analysis of covariance (ANCOVA) by using spm2. Age, sex, and total gray matter volume were included in the model as covariates to control for confounds. The level of statistical significance was set at P < 0.001, uncorrected, extent threshold 240 voxels (note that, in this high-resolution scan, one voxel is 1 mm3, one-eighth the size of the voxels in the functional data set).
Genotyping. Subjects were genotyped for the 5-HTT according to previously published protocols (22).
Results
Behavioral Performance. Analysis of reaction time (RT, in ms ± SD) data found no significant group differences in RTs in response to negative (S group, 689 ± 94 ms; L group, 719 ± 74 ms), neutral (S group, 676 ± 110 ms; L group, 687 ± 96 ms), or positive (S group, 704 ± 124 ms; L group, 700 ± 72 ms) words. A similar analysis of error rates (overall or for any valence condition) also yielded no significant differences as a function of 5-HTT genotype. Thus, group activation differences cannot be attributed to differences in task performance.
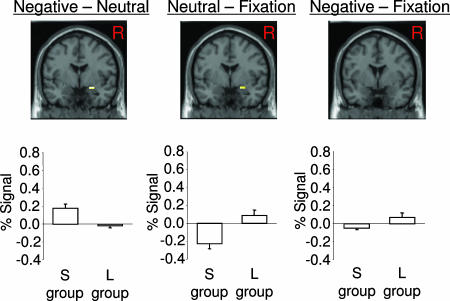
Amygdala Activation as a Function of 5-HTT Genotype. Fig. 1 displays amygdala activation as a function of 5-HTT genotype in response to negative, neutral, and fixation stimuli, using small-volume correction to control for multiple comparisons. There was significantly greater activation in the S group than the L group in response to negative, relative to neutral, words in the right amygdala (P < 0.05, false-discovery rate (FDR)-corrected; 21 voxels; peak voxel, P = 0.007, uncorrected, Montreal Neurological Institute (MNI) coordinates: 22, -2, -14), consistent with prior reports of significant amygdala activation to negative stimuli as a function of the short 5-HTT allele (3, 4, 6, 7). However, this effect may have been driven by the S group showing decreased activation in response to neutral stimuli, rather than increased activation to negative stimuli. To address this possibility, we used the fixation condition as a lower-level control. In support of this alternative explanation, we found that the S group exhibited significantly less activation than the L group in response to neutral, relative to fixation, stimuli in the right amygdala (P < 0.05, FDR-corrected; 22 voxels, peak voxel, P = 0.001; MNI coordinates, 22, 0, -18). Furthermore, there was no significant difference in amygdala activation between the S group and the L group in response to negative-fixation stimuli.
Fig. 1.
Amygdala activation to negative, neutral, and fixation stimuli. (Upper) Results of three separate contrast analyses, projecting significant clusters onto a coronal plane through the amygdala. (Lower) Corresponding bar graphs with percentage of signal change (±SEM) across the entire region of interest (ROI). A common ROI was used across all three analyses to extract individual mean activation values and was formed by the conjunction of the functionally defined ROIs in the negative-neutral and neutral-fixation contrasts (there was no significant cluster in the negative-fixation contrast).
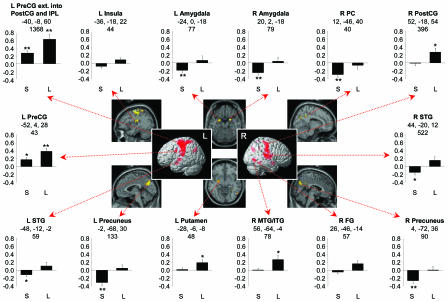
Whole-Brain Activation to Neutral Stimuli as a Function of 5-HTT Genotype. Fig. 2 illustrates regions in which activation to neutral words, relative to a fixation baseline, differed significantly between the S and L groups (a tabular listing of these regions is available in Table 2, which is published as supporting information on the PNAS web site). There were no clusters where the S group showed greater activation than the L group. In motor regions like the left precentral and postcentral gyri, both groups showed increased activation to neutral, relative to fixation, stimuli, but the L group showed significantly greater activation than the S group. In other regions, particularly right amygdala, bilateral precuneus, and posterior cingulate, the relative group differences is mostly driven by decreased activation in the S group to neutral, relative to fixation, stimuli.
Fig. 2.
Areas of significant group differences, as a function of 5-HTT genotype, to neutral words, compared with a fixation baseline condition. Bar graphs represent percentage of signal change (±SEM) across the entire cluster in the left (L) or right (R) hemisphere. The row of three numbers refers to Montreal Neurological Institute (MNI) coordinates of maximally significant voxel; the single number below refers to the cluster size in voxels. Significant differences from baseline are indicated by *, P < 0.05 and **, P < 0.01. PreCG, precentral gyrus; PostCG, postcentral gyrus; IPL, inferior parietal lobule; STG, superior temporal gyrus; MTG, middle temporal gyrus; ITG, inferior temporal gyrus; FG, fusiform gyrus; PC, posterior cingulate.
Whole-Brain Activation to Emotional Stimuli as a Function of 5-HTT Genotype. In our analysis of brain activation to emotional stimuli, we next chose a neutral word baseline to remove motor confounds across the stimulus conditions. Because we had already identified regions in which brain responses to neutral stimuli differed significantly as a function of genotype, we excluded these regions from the analysis.
For the negative-neutral contrast (Table 1, Negative-neutral), the S group showed significantly greater activation than the L group, which was most prominent in the insula, putamen, and caudate. In contrast, the L group did not show any regions of greater activation, compared with the S group. For the positive-neutral contrast (Table 1, Positive-neutral), the S group showed significantly greater activation than the L group, which was most prominent in the bilateral superior frontal gyrus (larger in the left hemisphere), as well as left posterior cingulate, inferior parietal lobule, and middle frontal gyrus. The L group showed significantly greater activation than the S group in the right precuneus.
Table 1. Significant activation to emotional-neutral stimuli.
| MNI coordinates
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Contrasts | Hemisphere | Anatomical location (BA) | x | y | z | Cluster size | T-score | P value |
| Negative-neutral, exclusively masked with neutral-fixation | ||||||||
| S > L | Left | Insula (BA 44/45), ext. into putamen | −40 | 8 | −14 | 1199 | 3.59 | 0.000 |
| Caudate, ext. into STG (BA 22) | −28 | −32 | 18 | 99 | 2.51 | 0.008 | ||
| Right | Putamen, ext. into insula (BA 44/45) | 30 | −6 | −8 | 1421 | 3.61 | 0.000 | |
| PreCG (BA 4) | 36 | −2 | 42 | 60 | 2.74 | 0.005 | ||
| MFG (BA 6) | 26 | −8 | 48 | 80 | 2.36 | 0.012 | ||
| MTG/ITG (BA 37) | 52 | −56 | −8 | 40 | 2.49 | 0.009 | ||
| IPS (BA 7/40) | 32 | −58 | 64 | 76 | 2.46 | 0.009 | ||
| Caudate, ext. into thalamus | 6 | 4 | 4 | 236 | 2.31 | 0.013 | ||
| AC (BA 32) | 10 | 18 | 42 | 54 | 2.24 | 0.015 | ||
| S < L | — | — | — | — | — | — | — | — |
| Positive-neutral, exclusively masked with neutral-fixation | ||||||||
| S > L | Left | SFG (BA 8/9) | −8 | 48 | 30 | 372 | 3.34 | 0.001 |
| IPL (BA 40) | −54 | −56 | 38 | 96 | 2.67 | 0.005 | ||
| MFG (BA 9) | −40 | 14 | 40 | 83 | 2.46 | 0.009 | ||
| MFG (BA 6/8) | −34 | 12 | 54 | 65 | 2.41 | 0.010 | ||
| MFG (BA 8/9) | −44 | 12 | 32 | 65 | 2.31 | 0.013 | ||
| IFG (BA 44/45) | −44 | 18 | 14 | 40 | 2.38 | 0.011 | ||
| PC (BA 31) | −6 | −50 | 40 | 245 | 2.32 | 0.013 | ||
| IPL (BA 7/40) | −40 | −46 | 62 | 64 | 2.31 | 0.013 | ||
| Right | SFG (BA 9) | 8 | 46 | 34 | 99 | 2.84 | 0.004 | |
| S < L | Left | — | — | — | — | — | — | — |
| Right | Precuneus (BA 7) | 16 | −38 | 56 | 48 | 2.38 | 0.011 | |
MNI, Montreal Neurological Institute; AC, anterior cingulate; BA, Brodmann's area; IFG, inferior frontal gyrus; IPL, inferior parietal lobule; IPS, intraparietal sulcus; MFG, middle frontal gyrus; MTG/ITG, middle temporal gyrus/inferior temporal gyrus; PC, posterior cingulate; PreCG, precentral gyrus; SFG, superior frontal gyrus; STG, superior temporal gyrus.x,y, and z coordinates, T-score, and P value apply to the most significant voxel in each cluster.
Structural Effects of 5-HTT Genotype. VBM analyses revealed that the S group had significantly greater volume in the left cerebellum, whereas the L group had significantly greater volume in a region of the left superior and medial frontal gyri, left anterior cingulate, and right inferior frontal gyrus. Analysis of the gray matter density found that the S group did not exhibit greater gray matter density than the L group anywhere in the brain. In contrast, the L group exhibited greater gray matter density than the S group bilaterally in the insula, left middle frontal gyrus and gyrus rectus, and right inferior temporal gyrus, anterior cingulate, and cerebellum. A full listing of regions is available in Table 3, which is published as supporting information on the PNAS web site.
Discussion
Individual differences in traits related to negative affect have been associated with presence of the 5-HTT short allele (1). Genomic imaging studies have concluded that this association may be due to increased amygdala activation to negative (relative to neutral) stimuli as a function of this allele (3, 4, 6, 7), based on the assumption that the neutral control condition itself would not generate activation differences as a function of 5-HTT genotype. We explicitly tested this assumption and found that brain response to neutral word stimuli differs as a function of the 5-HTT short allele in the amygdala, as well as other brain regions. We also showed that 5-HTT genotype is associated with activation changes to positive, in addition to negative, stimuli, and with structural changes in frontal, limbic, and cerebellar regions. The aggregate of these observations suggests that 5-HT transport efficiency has a much broader role in modulating brain function than previously thought.
Functional Considerations. Prior reports converged on the conclusion that the presence of the 5-HTT short allele is associated with greater activation to negative stimuli (3, 4, 6, 7). When we applied a similar comparison between a negative and a neutral word condition, we replicated this observation. Indeed, in a field cluttered with inconsistent amygdala laterality findings (32), we note the remarkable consistency of lateralized amygdala activation associated with the 5-HTT short allele. All studies (including ours) found significant amygdala activation to emotional stimuli as a function of 5-HTT genotype only in the right hemisphere, with the exception of Heinz et al. (7), who reported a bilateral effect. However, our conclusions about the nature of this activation differ from prior work. Based on comparisons with a fixation condition in amygdala small-volume-corrected analyses, our data show that greater amygdala activation to negative-neutral stimuli in 5-HTT short allele carriers is driven by decreased activation to neutral, rather than increased activation to negative, stimuli (both relative to a fixation condition; see Fig. 1). This finding is consistent with a metaanalysis of nine PET studies that also reported consistent decreases in right amygdala activation across several active, relative to passive, tasks (33).
Two interpretations can be derived from this observation. Carriers of the 5-HTT short allele may either show decreased activation during the active (neutral word) task or increased activation during the passive (fixation) task (33). As Shulman et al. suggest (33), increased activity during the passive condition could reflect ongoing processes, such as constant vigilance (34), that are suspended during the active task. At the neural level, this interpretation would suggest that 5-HTT short allele carriers may be characterized by tonic amygdala activation during rest, as opposed to phasic amygdala deactivation during active task performance. In humans, future work using event-related fMRI could address this possibility. Alternatively, future electrophysiological studies in transgenic animals could conduct in vivo recordings to determine whether the amygdala exhibits tonic increases in activation, compared with wild-type animals, as a function of reduced serotonin transporter availability.
When we conducted a whole-brain analysis, based on a contrast between the neutral and fixation condition (see Fig. 2), carriers of the 5-HTT short allele (S group) consistently showed less activation than non-carriers (L group). In some regions, this group difference was driven by decreased activation to the neutral condition in the S group. In addition to the amygdala (already discussed above), such regions included two medium-sized clusters in the precuneus and one cluster at the minimum-size threshold that was located in the right posterior cingulate. These regions were also noted in PET studies that identified areas of decreased activation across several active task, relative to passive rest, conditions (33, 35). Another was located in the superior temporal gyrus, which has not been reported in these previous PET analyses and may therefore reflect task-dependent processes.
In other regions, the relative activation difference between the S and L groups was expressed in increased activation to the neutral condition in both groups; with the L group expressing significantly greater activation than the S group (Fig. 2). These were motor regions, most prominently the left primary motor and primary somatosensory areas. Activation of these regions is consistent with the fact that subjects used their right hand in the active (neutral word) task and did not produce a motor response during the fixation task. Smaller motor cortex activation in carriers of the 5-HTT short allele is consistent with the modulatory role of serotonin in motor processes (36). To the extent that the short allele is associated with depression (37), and depression with psychomotor retardation (38), our data are also consistent with a recent report (39) that depressed patients who carry the 5-HTT short allele show less nocturnal motor behavior than non-carriers.
We now turn the discussion to the differential effects of 5-HTT genotype on brain reactivity to emotional, relative to neutral, word stimuli. These analyses were conducted in a manner to exclude regions where the S and L groups differed in response to neutral (relative to fixation) stimuli. For the analysis based on a contrast between negative and neutral words (see Table 1, Negative-neutral), the S group showed greater activation than the L group in nine clusters (totaling 3,265 significant voxels), seven of which (1,967 significant voxels, or 60%) were lateralized to the right hemisphere. The largest activation (accounting for 2,620 voxels, or 80% of the total number of significant voxels) was observed bilaterally (more voxels in the right) in a region that included the insula and putamen. Another large cluster was located in the caudate, extending into thalamus. Insula activation has been associated with visceral feeling responses (40), response to emotional processing during cognitive load (41), and may play a role in monitoring the internal emotional state (40) or distressing cognitions (42). Activation in putamen, caudate, and thalamus to negative stimuli is consistent with a study that has linked these regions to emotional distress (43). There were no clusters where the L group showed greater activation than the S group. This pattern of activation is consistent with prior reports highlighting a role for the 5-HTT short allele in brain reactivity to negative stimuli (3-7) and with models that posit right-hemispheric specialization for the processing of negative stimuli (44).
For the analysis based on a contrast between positive and neutral words (see Table 1, Positive-neutral), the S group showed greater activation than the L group in nine clusters (totaling 1,129 significant voxels), eight of which (1,030 significant voxels, or 91%) were lateralized to the left hemisphere. The largest of these clusters was located in the left superior frontal gyrus and posterior cingulate. In contrast, the L group showed greater activation in only one small cluster (48 voxels), at modest significance levels, in the right precuneus. There were no group differences in amygdala activation, consistent with the only other study to use positive emotional stimuli with 5-HTT-genotyped fMRI participants (7). The strong left-lateralization in brain response to positive stimuli is consistent with models that posit left-hemispheric specialization for the processing of positive stimuli (44). The data show that presence of the 5-HTT short allele is not limited to modulating neural responses to negative, but also to positive stimuli, suggesting a role in emotional arousal in general, rather than negative valence specifically.
Structural Considerations. 5-HTT genotype also conferred structural differences. By analyzing high-resolution structural scans, we found that 5-HTT genotype affects brain volume and gray matter density, primarily in frontal, limbic, and cerebellar regions.
The S group showed more volume than the L group in the left cerebellum, but less gray matter density in the right cerebellum. Although the significance of this observation is currently unclear, both functional and structural abnormalities have been reported in the cerebellum. Increased blood flow has been reported in the cerebellum in patients with major depressive disorder (MDD, refs. 45 and 46), and during induced negative affect (47). Damage to the cerebellum has been associated with a complex set of behavioral changes coined the “cerebellar cognitive affective syndrome” (48).
We found evidence for significantly smaller volume and reduced gray matter density in the middle frontal gyrus [Brodmann's area (BA) 9] in the S group, compared with the L group. This observation is consistent with a report that patients with major depressive disorder show a significant reduction in neurons and glia in this region (49). We also found decreased gray matter density in the S group, compared with the L group, in the gyrus rectus, a region previously noted to be decreased in volume in depressed patients (50).
The S group showed decreased volume and gray matter density in the anterior cingulate (AC), compared with the L group. Decreased volume was seen in the affective division of the AC, whereas decreased gray matter density was seen in the pregenual region, consistent with another report (5).
Study Limitations and Statistical Considerations. Our conclusions regarding the role of 5-HTT in the amygdala rest on analyses that used small-volume corrections in this a priori region of interest. The fact that we replicated these results when using a whole-brain analysis approach (see below) further demonstrates the robustness of our finding. Nonetheless, our observations are currently limited to the emotional word Stroop paradigm. Studies that have reported an effect of 5-HTT variation on amygdala activation have used a facial matching task (3-5), a verbal task (6), and passive viewing of complex pictures (7). The fact that this variety of tasks converges on the amygdala as a pivotal region to be modulated by 5-HTT genotype suggests a broad role in affective processing, independent of task conditions. Nonetheless, our study was motivated by the fact that none of these prior studies used a lower-level passive control condition to test the assumption that the neutral control condition has no effect. Thus, future work should modify these paradigms to include a passive rest condition, to test the generalizability of our conclusions.
Future work also should address whether affective state or level of processing (implicit versus explicit) acts to modulate the observed effects. For example, it is possible that the presence of negative or positive stimuli generated a mood state that affected processing of stimuli during neutral blocks. However, prior reports have been consistent regardless of whether subjects were exposed to an explicit mood induction procedure (6) or not (3-5, 7), but these observations were made across tasks. Future work should vary instructions within the same task to compare amygdala activation as a function of 5-HTT genotype under explicit and implicit emotion-processing conditions.
The challenge faced in any whole-brain analysis is that the sheer number of voxels and the lack of full independence across voxels make it difficult to apply a Bonferroni correction to protect against false positive errors. A full Bonferroni correction would lead to a substantial loss of statistical power to detect statistically significant activation. An alternative approach has been proposed that allows reasonable protection from false positive activations by incorporating statistical thresholds with conjunction of spatial extent or cluster-size thresholds (28, 51). This second perspective is based on the assumption that true neural activity, as measured with functional neuroimaging methods, should produce signal changes that are larger than individual voxels and extend over contiguous voxels. Forman et al. (28) used Monte Carlo simulations and fMRI data to show that this approach can improve statistical power by as much as 5-fold over methods that rely on activation thresholds alone.
For our whole-brain functional analyses, we used an activation threshold of P < 0.05 with a cluster size threshold of 40 voxels, to achieve a per-pixel false positive correction of ≈P < 0.0008 (based on extrapolation from ref. 28). Note that most of the clusters we reported are much larger than the required minimum, several by one order of magnitude or more. We therefore feel that the activation differences we report between carriers and non-carriers of the 5-HTT short allele are appropriately controlled for false positive errors, and thus reflect true neural activation differences. Nonetheless, our whole-brain analyses were not constrained by a priori expectations and therefore have to await future replication attempts.
In conclusion, we have demonstrated that the inclusion of a lower-level control condition (passive rest) significantly alters the conclusion we draw with respect to the role of 5-HTT genetic variation in amygdala function. Rather than driving increased reactivity to negative stimuli, presence of the 5-HTT short allele is associated with decreased activation to neutral stimuli during the emotional word Stroop task. If this observation generalizes across other paradigms, future work will need to expend more effort to reveal the neural mechanisms by which reduced serotonin transporter availability (associated with the 5-HTT short allele) produces either phasic decreases in amygdala activation across different active conditions, or tonic increases during the passive rest condition.
Supplementary Material
Acknowledgments
We thank N. Steigerwald for excellent technical assistance in DNA sample processing and genotyping, and J. Ferri for excellent assistance in collecting fMRI data. This work was supported by Stony Brook University, General Clinical Research Center Grant 5-MO1-RR-10710, National Science Foundation Grant BCS-0224221, European Commission Grant NEWMOOD LSHM-CT-2003-503474, Deutsche Forschungsgemeinschaft Grants SFB 581 and KFO 125/1-1, and Bundesministerium für Bildung und Forschung Grant IZKF 01 KS 9603.
Author contributions: T.C. designed research; T.C. performed research; T.C., K.O., B.W.H., and A.F. analyzed data; R.T.C. designed fMRI pulse sequence; and T.C., R.T.C., and K.P.L. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: fMRI, functional MRI; PET, positron-emission tomography; VBM, voxel-based morphometry; L group, long variant group; S group, short variant group.
References
- 1.Lesch, K. P. (2004) J. Psychiatry Neurosci. 29, 174-184. [PMC free article] [PubMed] [Google Scholar]
- 2.Sen, S., Burmeister, M. & Ghosh, D. (2004) Am. J. Med. Genet. 127B, 85-89. [DOI] [PubMed] [Google Scholar]
- 3.Hariri, A. R., Mattay, V. S., Tessitore, A., Kolachana, B., Fera, F., Goldman, D., Egan, M. F. & Weinberger, D. R. (2002) Science 297, 400-403. [DOI] [PubMed] [Google Scholar]
- 4.Hariri, A. R., Drabant, E. M., Munoz, K. E., Kolachana, B. S., Mattay, V. S., Egan, M. F. & Weinberger, D. R. (2005) Arch. Gen. Psychiatry 62, 146-152. [DOI] [PubMed] [Google Scholar]
- 5.Pezawas, L., Meyer-Lindenberg, A., Drabant, E. M., Verchinski, B. A., Munoz, K. E., Kolachana, B. S., Egan, M. F., Mattay, V. S., Hariri, A. R. & Weinberger, D. R. (2005) Nat. Neurosci. 8, 828-834. [DOI] [PubMed] [Google Scholar]
- 6.Furmark, T., Tillfors, M., Garpenstrand, H., Marteinsdottir, I., Langstrom, B., Oreland, L. & Fredrikson, M. (2004) Neurosci. Lett. 362, 189-192. [DOI] [PubMed] [Google Scholar]
- 7.Heinz, A., Braus, D. F., Smolka, M. N., Wrase, J., Puls, I., Hermann, D., Klein, S., Grusser, S. M., Flor, H., Schumann, G., et al. (2005) Nat. Neurosci. 8, 20-21. [DOI] [PubMed] [Google Scholar]
- 8.Pardo, J. V., Pardo, P. J., Janer, K. W. & Raichle, M. E. (1990) Proc. Natl. Acad. Sci. USA 87, 256-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Canli, T., Amin, Z., Haas, B., Omura, K. & Constable, R. T. (2004) Behav. Neurosci. 118, 897-904. [DOI] [PubMed] [Google Scholar]
- 10.Canli, T., Zhao, Z., Desmond, J. E., Kang, E., Gross, J. & Gabrieli, J. D. E. (2001) Behav. Neurosci. 115, 33-42. [DOI] [PubMed] [Google Scholar]
- 11.Bush, G., Whalen, P. J., Rosen, B. R., Jenike, M. A., McInerney, S. C. & Rauch, S. L. (1998) Hum. Brain Mapp. 6, 270-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whalen, P. J., Bush, G., McNally, R. J., Wilhelm, S., McInerney, S. C., Jenike, M. A. & Rauch, S. L. (1998) Biol. Psychiatry 44, 1219-1228. [DOI] [PubMed] [Google Scholar]
- 13.Isenberg, N., Silbersweig, D., Engelien, A., Emmerich, S., Malavade, K., Beattie, B., Leon, A. C. & Stern, E. (1999) Proc. Natl. Acad. Sci. USA 96, 10456-10459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allman, J. M., Hakeem, A., Erwin, J. M., Nimchinsky, E. & Hof, P. (2001) Ann. N.Y. Acad. Sci. 935, 107-117. [PubMed] [Google Scholar]
- 15.Bush, G., Luu, P. & Posner, M. I. (2000) Trends Cogn. Sci. 4, 215-222. [DOI] [PubMed] [Google Scholar]
- 16.Davidson, R. J., Pizzagalli, D., Nitschke, J. B. & Putnam, K. (2002) Annu. Rev. Psychol. 53, 545-574. [DOI] [PubMed] [Google Scholar]
- 17.Devinsky, O., Morrell, M. J. & Vogt, B. A. (1995) Brain 118, 279-306. [DOI] [PubMed] [Google Scholar]
- 18.Mayberg, H. S. (1997) J. Neuropsychiatry Clin. Neurosci. 9, 471-481. [DOI] [PubMed] [Google Scholar]
- 19.Posner, M. I. & Rothbart, M. K. (1998) Philos. Trans. R. Soc. London B. Biol. Sci. 353, 1915-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davidson, R. J., Lewis, D. A., Alloy, L. B., Amaral, D. G., Bush, G., Cohen, J. D., Drevets, W. C., Farah, M. J., Kagan, J., McClelland, J. L., et al. (2002) Biol. Psychiatry 52, 478-502. [DOI] [PubMed] [Google Scholar]
- 21.Drevets, W. C. (2000) Prog. Brain Res. 126, 413-431. [DOI] [PubMed] [Google Scholar]
- 22.Lesch, K.-P., Bengel, D., Heils, A., Sabol, S. Z., Greenberg, B. D., Petri, S., Benjamin, J., Muller, C. R., Hamer, D. H. & Murphy, D. L. (1996) Science 274, 1527-1531. [DOI] [PubMed] [Google Scholar]
- 23.Lesch, K. P., Greenberg, B. D., Bennett, A., Higley, J. D. & Murphy, D. L. (2002) in Molecular Genetics and the Human Personality, eds. Benjamin, J., Ebstein, R. & Belmaker, R. H. (American Psychiatric Press, Washington, DC), pp. 109-135.
- 24.David, S. P., Murthy, N. V., Rabiner, E. A., Munafo, M. R., Johnstone, E. C., Jacob, R., Walton, R. T. & Grasby, P. M. (2005) J. Neurosci. 25, 2586-2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bradley, M. M. & Lang, P. J. (1999) Affective Norms for English Words (ANEW) (NIMH Center for the Study of Emotion and Attention, University of Florida, Gainesville).
- 26.Friston, K. J. (1994) in Functional Neuroimaging: Technical Foundations, ed. Thatcher, R. (Academic, San Diego), pp. 79-93.
- 27.Holmes, A. P. & Friston, K. J. (1998) NeuroImage 7, S754. [DOI] [PubMed] [Google Scholar]
- 28.Forman, S. D., Cohen, J. D., Fitzgerald, M., Eddy, W. F., Mintun, M. A. & Noll, D. C. (1995) Magn. Reson. Med. 33, 636-647. [DOI] [PubMed] [Google Scholar]
- 29.Ashburner, J. & Friston, K. J. (2001) NeuroImage 14, 1238-1243. [DOI] [PubMed] [Google Scholar]
- 30.Ashburner, J. & Friston, K. J. (2000) NeuroImage 11, 805-821. [DOI] [PubMed] [Google Scholar]
- 31.Good, C. D., Johnsrude, I. S., Ashburner, J., Henson, R. N., Friston, K. J. & Frackowiak, R. S. (2001) NeuroImage 14, 21-36. [DOI] [PubMed] [Google Scholar]
- 32.Baas, D., Aleman, A. & Kahn, R. S. (2004) Brain Res. Brain Res. Rev. 45, 96-103. [DOI] [PubMed] [Google Scholar]
- 33.Shulman, G. L., Fiez, J. A., Corbetta, M., Buckner, R. L., Miezin, F. M., Raichle, M. E. & Petersen, S. E. (1997) J. Cognit. Neurosci. 9, 648-663. [DOI] [PubMed] [Google Scholar]
- 34.Davis, M. & Whalen, P. J. (2001) Mol. Psychiatry 6, 13-34. [DOI] [PubMed] [Google Scholar]
- 35.Raichle, M. E., MacLeod, A. M., Snyder, A. Z., Powers, W. J., Gusnard, D. A. & Shulman, G. L. (2001) Proc. Natl. Acad. Sci. USA 98, 676-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Risch, N. & Merikangas, K. (1996) Science 273, 1516-1517. [DOI] [PubMed] [Google Scholar]
- 37.Caspi, A., Sugden, K., Moffitt, T. E., Taylor, A., Craig, I. W., Harrington, H., McClay, J., Mill, J., Martin, J., Braithwaite, A. & Poulton, R. (2003) Science 301, 386-389. [DOI] [PubMed] [Google Scholar]
- 38.American Psychiatric Association (2000) Diagnostic and Statistical Manual of Mental Disorders (Am. Psychiatr. Assoc., Washington, DC), 4th Ed., Text Revision.
- 39.Putzhammer, A., Schoeler, A., Rohrmeier, T., Sand, P., Hajak, G. & Eichhammer, P. (2005) Psychopharmacology 178, 303-308. [DOI] [PubMed] [Google Scholar]
- 40.Damasio, A. R., Grabowski, T. J., Bechara, A., Damasio, H., Ponto, L. L. B., Parvizi, J. & Hichwa, R. D. (2000) Nat. Neurosci. 3, 1049-1056. [DOI] [PubMed] [Google Scholar]
- 41.Phan, K. L., Wager, T., Taylor, S. F. & Liberzon, I. (2002) NeuroImage 16, 331-348. [DOI] [PubMed] [Google Scholar]
- 42.Reiman, E. M., Lane, R. D., Ahern, G. L., Schwartz, G. E., Davidson, R. J., Friston, K. J., Yun, L.-S. & Chen, K. (1997) Am. J. Psychiatry 154, 918-925. [DOI] [PubMed] [Google Scholar]
- 43.Sinha, R., Lacadie, C., Skudlarski, P. & Wexler, B. E. (2004) Ann. N.Y. Acad. Sci. 1032, 254-257. [DOI] [PubMed] [Google Scholar]
- 44.Davidson, R. J. (1995) in Brain Asymmetry, eds. Davidson, R. J. & Hugdahl, K. (MIT Press, Cambridge, MA).
- 45.Bench, C. J., Friston, K. J., Brown, R. G., Scott, L. C., Frackowiak, R. S. & Dolan, R. J. (1992) Psychol. Med. 22, 607-615. [DOI] [PubMed] [Google Scholar]
- 46.Buchsbaum, M. S., Wu, J., Siegel, B. V., Hackett, E., Trenary, M., Abel, L. & Reynolds, C. (1997) Biol. Psychiatry 41, 15-22. [DOI] [PubMed] [Google Scholar]
- 47.George, M. S., Ketter, T. A., Parekh, P. I., Horwitz, B., Herscovitch, P. & Post, R. M. (1995) Am. J. Psychiatry 152, 341-351. [DOI] [PubMed] [Google Scholar]
- 48.Schmahmann, J. D. & Sherman, J. C. (1998) Brain 121, 561-579. [DOI] [PubMed] [Google Scholar]
- 49.Rajkowska, G., Miguel-Hidalgo, J. J., Wei, J., Dilley, G., Pittman, S. D., Meltzer, H. Y., Overholser, J. C., Roth, B. L. & Stockmeier, C. A. (1999) Biol. Psychiatry 45, 1085-1098. [DOI] [PubMed] [Google Scholar]
- 50.Bremner, J. D., Vythilingam, M., Vermetten, E., Nazeer, A., Adil, J., Khan, S., Staib, L. H. & Charney, D. S. (2002) Biol. Psychiatry 51, 273-279. [DOI] [PubMed] [Google Scholar]
- 51.Friston, K. J., Worsley, K. J., Frackowiak, R. S. J., Mazziotta, J. C. & Evans, A. C. (1994) Hum. Brain Mapp. 1, 210-220. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.