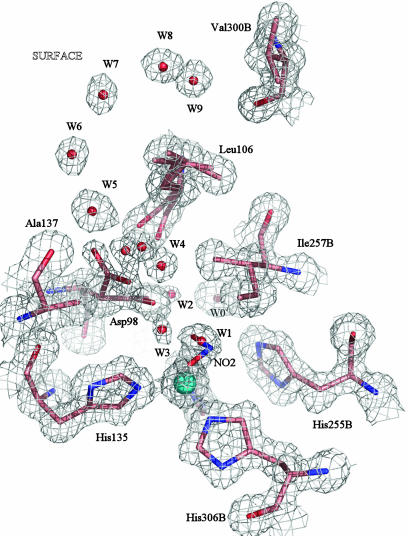
Fig. 2.
The T2Cu site of the nitrite-soaked enzyme at 1.10 Å resolution, shown with 2Fobs – Fcalc electron density contoured at 1.2σ. Either a water or nitrite molecule occupies the substrate-binding site, with the nitrite OD2/OD1 atoms at 1.98/2.19 Å and the N atom at 2.15 Å or a water molecule, W1 at 2.0 Å from T2Cu. The B factors of the Cu atom and nitrite ligand are 10.1 Å2 and 16.0 Å2, respectively, compared with the B factors for ligating N(His), which average 10.0 Å2. The Cu occupancy of the protein was 90%. The nitrite is oriented so that the O1 and O2 atoms form H-bonds with water W2 at 2.4 Å and with water W3, at 2.6 Å. W2 is 2.4 Å from the conserved proton channel water molecule, W0. The substrate-entry water network of the resting-state enzyme is conserved in this structure, while the gatekeeper conformation of the Asp-98 is more pronounced in the electron density map.