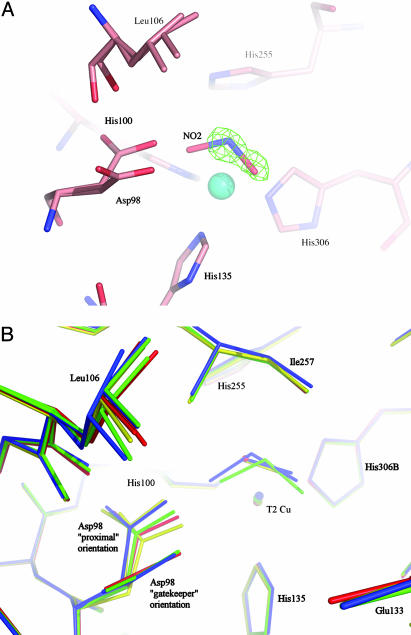
Fig. 4.
Comparison of T2Cu site in resting, nitrite-bound, NO bound, or mixed (NO and NO2) forms. (A) The catalytic T2 Cu site of AcNiR with endogenously bound nitrite and NO in the same crystal, with the NO removed. The nitrite ligand has been modeled in the 2Fobs – Fcalc electron density map, while the presence of the NO ligand is evident in the resulting Fobs – Fcalc difference electron density map, contoured at 3.2σ. (B) Comparison of the T2Cu site of the enzyme in the resting state (red), nitrite soaked (green), endogenously bound NO (yellow), and endogenously bound nitrite and NO trapped in the same crystal (blue). A perspective view is shown from the surface of the protein into the substrate-entry channel. For clarity, all water molecules except the bound water in the resting state have been omitted. The flexibility of the Leu-106 residue and the nearly 90° flip of the Asp-98 side chain, between the gatekeeper orientation (pointing out of the page) and the proximal orientation, are clearly illustrated.