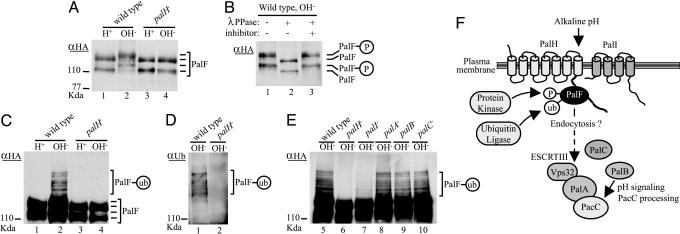
Fig. 4.
PalH- and alkaline ambient pH-dependent phosphorylation and ubiquitination of PalF: A model for pH signaling in A. nidulans. (A-E) WT and indicated pal mutants expressing PalF-(HA)3 were grown under acidic conditions (H+) and shifted for 30 min to alkaline conditions (OH-). Total (A) or anti-(α)-HA-immunoprecipitated (B-E) protein extracts were analyzed by Western blotting. Positions of the different PalF forms (P, phosphorylated; ub, ubiquitinated) are indicated. (A) Immunoblot of extracts with anti-HA Ab. (B) Anti-HA immunoprecipitates from the WT shifted to alkaline conditions were treated with λ phosphatase (λPPase), with or without phosphatase inhibitor. (C and E) Anti-HA immunoprecipitates were immunoblotted with anti-HA Ab. (D) Anti-HA immunoprecipitates were immunoblotted with anti-ubiquitin Ab. pH signaling is impaired in all pal strains, as determined by deficient PacC proteolytic processing at alkaline pH (data not shown). (F) Model for pH signaling in A. nidulans. The predicted 7TM PalH would be a pH sensor activating the signaling pathway possibly with assistance of 4TM protein PalI, in agreement with the accessory role suggested by partial impairment of pH signaling in palI-null mutants (ref. 7 and this work). PalF interacts with the PalH cytoplasmic tail and is phosphorylated and ubiquitinated at alkaline pH by as-yet-unidentified protein kinase and ubiquitin ligase enzymes in a PalH-dependent and partially PalI-dependent manner. Based on the role that β-arrestin dephosphorylation and ubiquitination play in receptor-mediated endocytosis, we propose that PalF phosphorylation/ubiquitination promotes internalization of PalF-PalH complexes to transduce the pH signal to the PalA/PalB PacC-processing machinery acting downstream of PalF (this work). PalA/Rim20 interacts with PacC/Rim101 to mediate its signaling proteolytic cleavage, presumably by the cysteine protease PalB/Rim13 (9-11), and with Vps32, a component of the ESCRT complexes transiently associating to endosomal membranes (10, 12).