Table 5.
Preclinical studies of targeting other molecular chaperones
| Compound | Structure | Activity | Mechanism (Sites) |
|---|---|---|---|
| Compound 2H |  |
cIC50 = 5.0 µM | HSP110 Inhibitor (/) |
| iHSP110-33 | 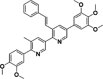 |
bIC50 = 58 μM | HSP110 Inhibitor (NBD) |
| GdnHCl |  |
Kd ≈ 600 μM | ClpB Inhibitor (NTD) |
| DBeQ |  |
aIC50 ≈ 5.0 μM | ClpB Inhibitor (NTD) |
| Mizoribine |  |
/ | HSP60 inhibitor (NBD) |
| ETB |  |
cIC50 = 1.1 μM | HSP60 inhibitor (Cys442 covalent site) |
| MC |  |
/ | HSP60 inhibitor (/) |
| O-carboranyl-phenoxyacetanilide | 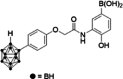 |
cIC50 = 0.74 μM | HSP60 inhibitor (NBD) |
| [Au(TPP)Cl] |  |
cIC50 = 1.1 μM | HSP60 inhibitor (/) |
| KHS101 |  |
cIC50 = 14 μM | HSP60 inhibitor (/) |
| RP101 |  |
/ | HSP27 inhibitor (Phe29 and Phe33) |
| J2 | 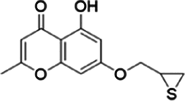 |
cIC50 = 99 μM | HSP27 inhibitor (Cysteine-thiol group) |
| MK-933 |  |
/ | HSP27 inhibitor (NTD) |
Kd target binding affinity, aIC50 ATPase inhibitor activity, bIC50 competitive binding activity, cIC50 inhibitory activity on molecular chaperone functions, “/” indicates no clear reports
