Table 4.
Derivatization of halogen-substituted N-acetyl diazocines via Suzuki–Miyaura reaction. Equivalents are normalized to the used amount of N-acetyl diazocine starting material.

| ||
|
| ||
| boronic acid | product | yield |
|
| ||

|

7 |
X = Br, 74% X = I, 83% |

|

9 |
X = Br, 68% X = I, 74% |

|

10 |
X = I, 82% |

|

11 |
X = Br, 81% X = I, 88% |

|

12 |
X = Br, 70% X = I, 77% |

|

13 |
X = Br, 76%a X = I, 79%a |

|

14 |
X = Br, 7% X = I, 19% |

|

15 |
X = Br, – X = I, – |

|

16 |
X = Br, – X = I, – |

|

17 |
X = Br, 45% X = I, – |
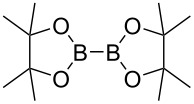
|

18 |
X = Br, – X = I, – |
aThe reaction was carried out in dry DMF at 100 °C because no reaction took place if the Suzuki–Miyaura standard procedure was applied.
