Abstract
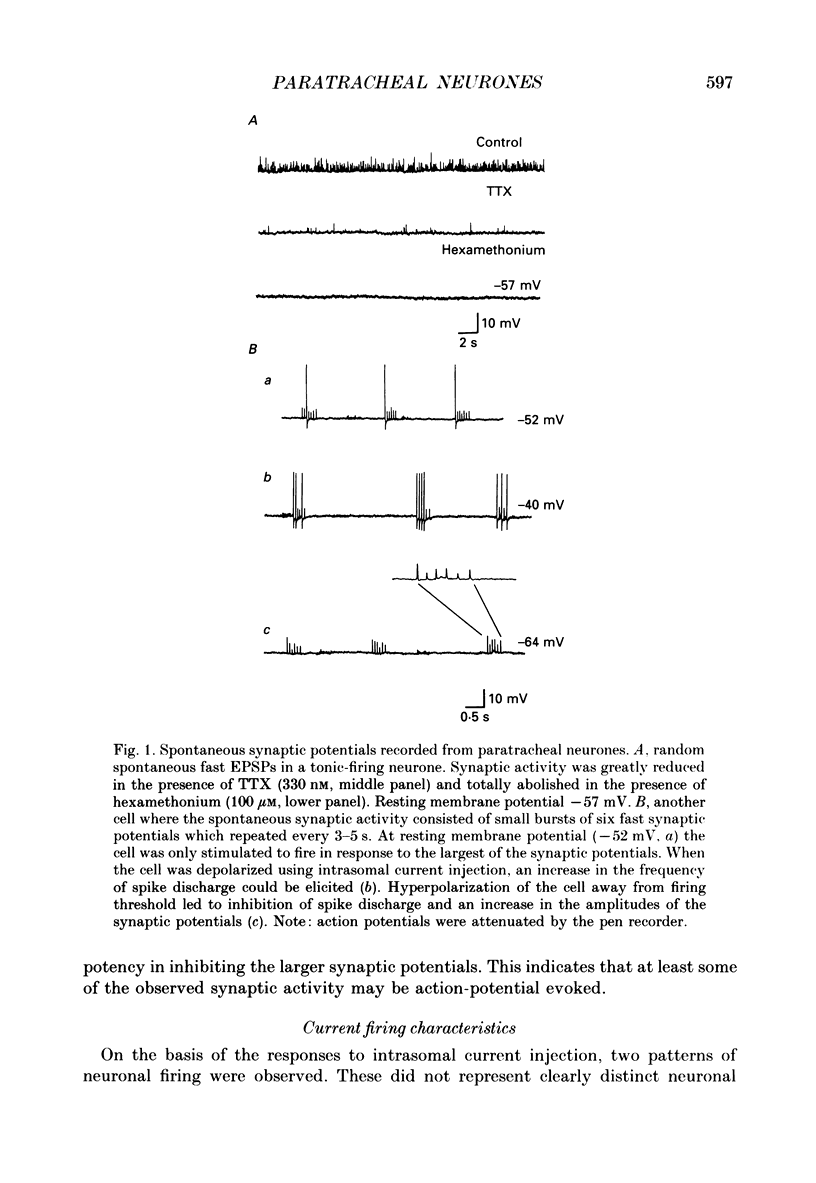
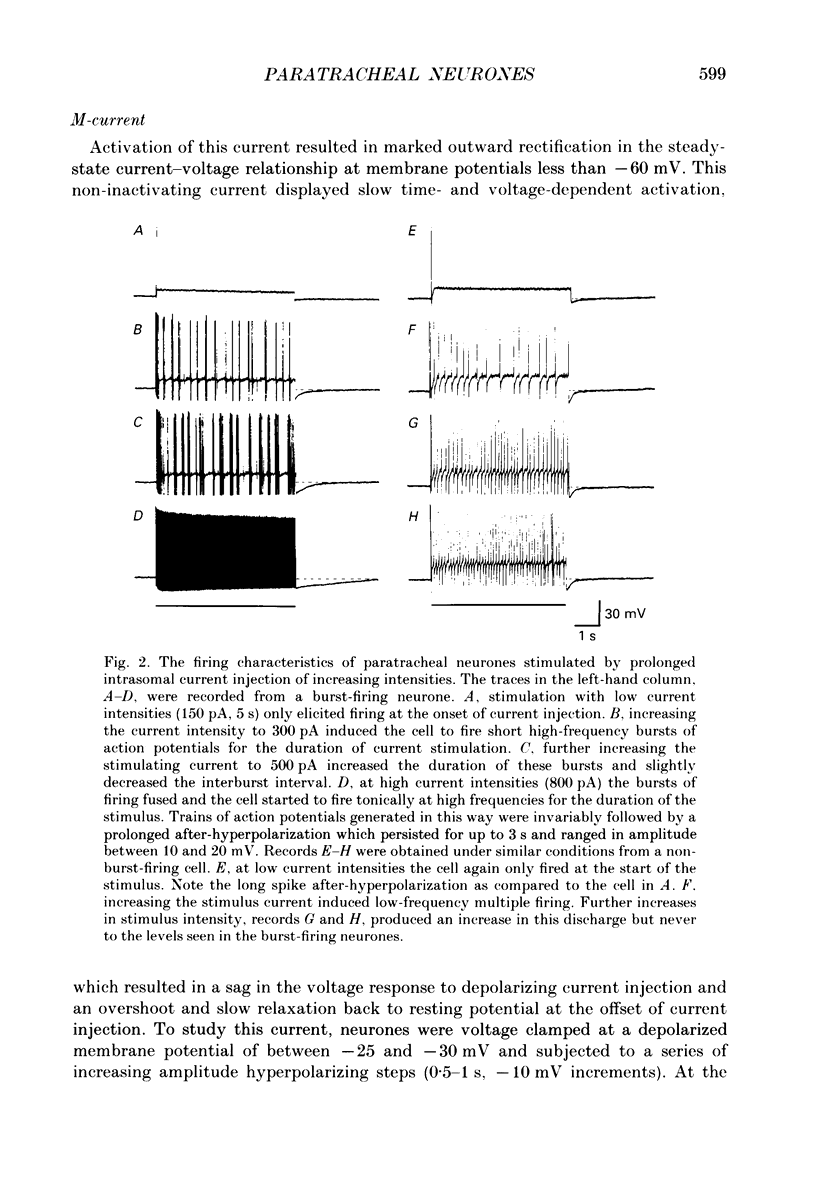
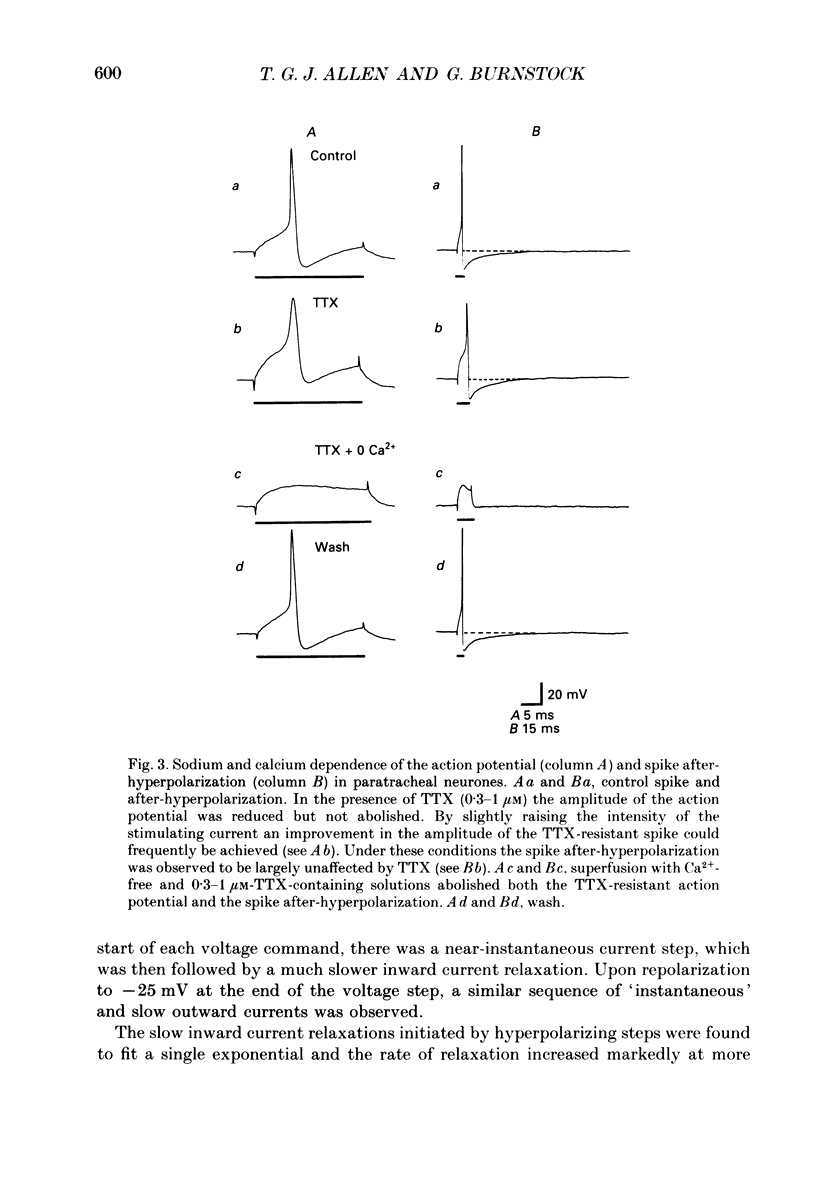
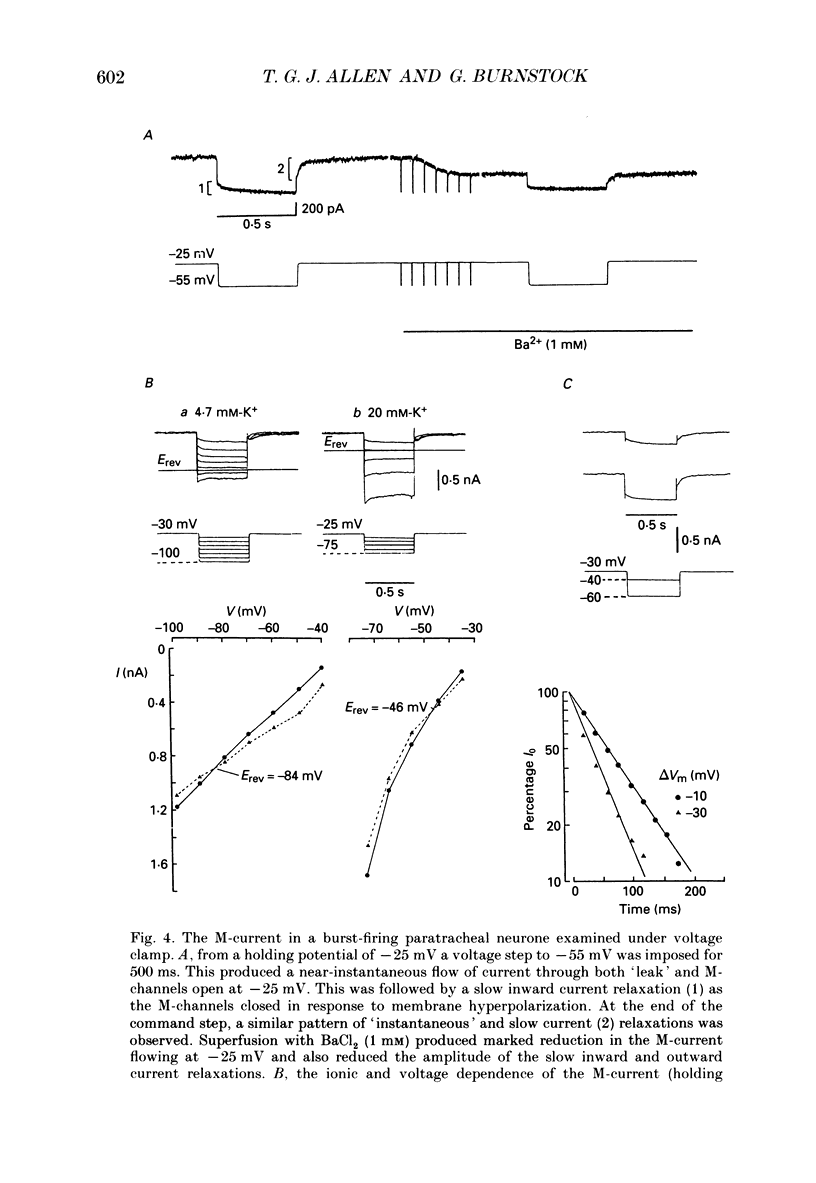
1. The electrophysiological characteristics of intramural neurones from the paratracheal ganglia of 14- to 18-day-old rats were studied in vitro using intracellular, single-electrode current- and voltage-clamp techniques. 2. Resting membrane potentials ranged between -50 and -73 mV. In 50-60% of all neurones, random and occasionally patterned bursts of spontaneous, fast synaptic potentials were observed. In all cases, superfusion with either hexamethonium (100 microM), or Ca2(+)-free, high-magnesium-containing solutions abolished all synaptic activity. 3. Two distinct patterns of spike discharge were observed in response to prolonged intrasomal current injection. Most cells (65-75%) fired rhythmic, high-frequency (50-90 Hz) bursts of action potentials, with interburst intervals of between 300 and 500 ms, throughout the period of current stimulation. A further 10-15% of cells fired tonically at low frequencies (10-15 Hz) for the duration of the applied stimulus. In both cell types, trains of action potentials were followed by a pronounced calcium-dependent after-hyperpolarization which persisted for up to 3 s. The magnitude of the after-hyperpolarization following a single spike in tonic-firing cells was considerably larger than in burst-firing cells. Both the action potential and the after-hyperpolarization in all cells displayed a calcium-dependent, tetrodotoxin-resistant component which was abolished by the removal of the extracellular calcium. 4. The spike after-hyperpolarization resulted from activation of an outward calcium-dependent potassium current which reversed at -86.5 mV. This value was shifted by 63.6 mV for a 10-fold increase in extracellular potassium concentration. 5. All of the cells studied exhibited marked outward rectification when depolarized. This resulted from activation of a time- and voltage-dependent M-current. The slow inward current relaxations associated with the M-current became faster at more negative potentials and reversed around -85 mV. Raising the extracellular potassium concentration shifted the reversal potential for the current relaxations to more depolarized potentials in a manner predicted by the Nernst equation for a current carried by potassium ions. Both the outward current at depolarized potentials and the slow current relaxations were potently inhibited by extracellular BaCl2 (1 mM) but were unaffected by CsCl (1-3 mM). 6. Inward rectification at hyperpolarized potentials was a characteristic of all cells. Membrane hyperpolarization revealed inward rectification in the 'instantaneous' current-voltage relationship at membrane potentials greater than -80 mV.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
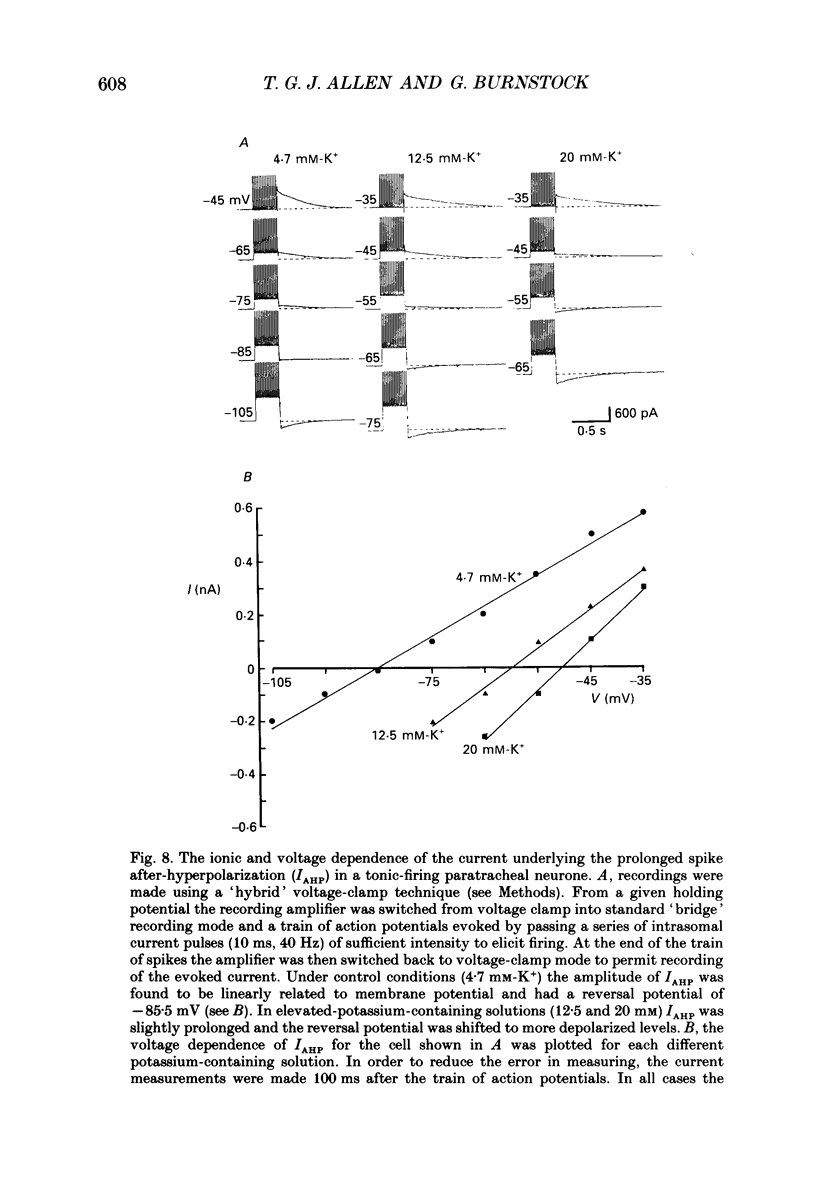
PDF












Selected References
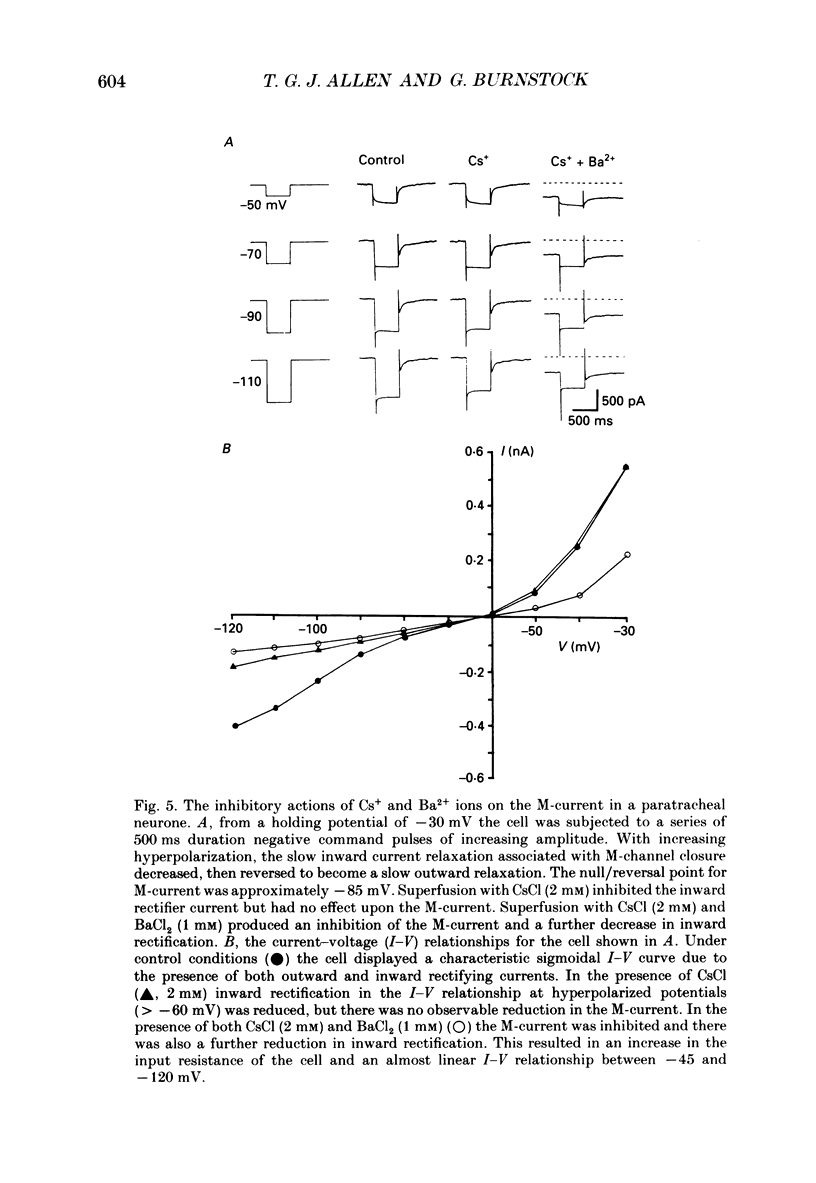
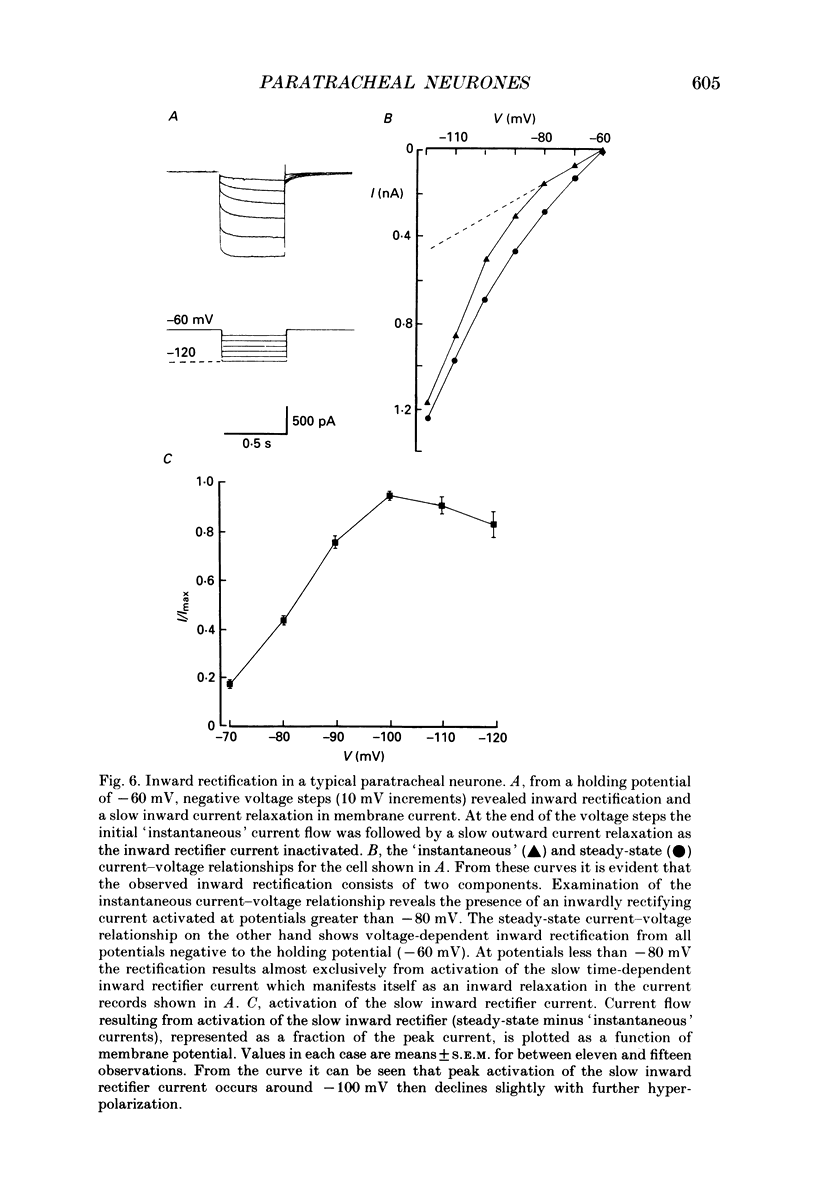
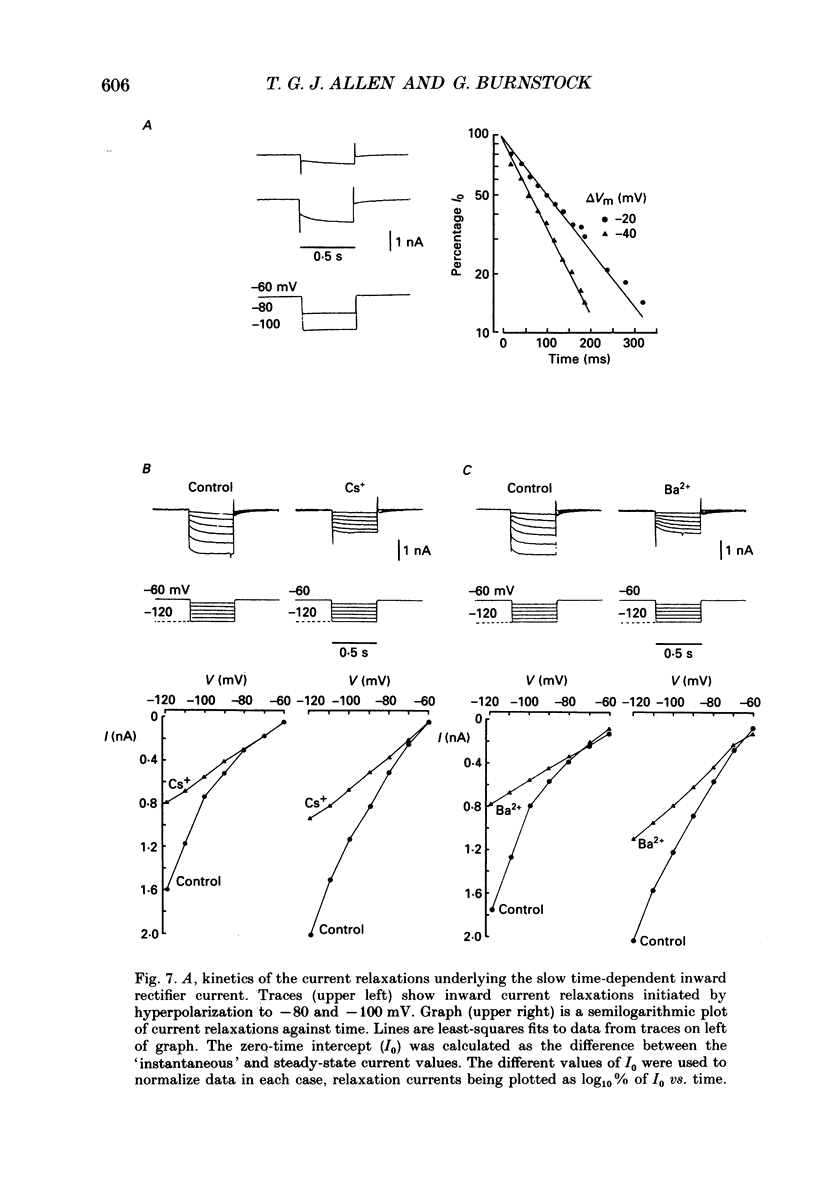
These references are in PubMed. This may not be the complete list of references from this article.
- Allen T. G., Burnstock G. Intracellular studies of the electrophysiological properties of cultured intracardiac neurones of the guinea-pig. J Physiol. 1987 Jul;388:349–366. doi: 10.1113/jphysiol.1987.sp016618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D. G., McDonald D. M., Basbaum C. B., Mitchell R. A. The architecture of nerves and ganglia of the ferret trachea as revealed by acetylcholinesterase histochemistry. J Comp Neurol. 1986 Apr 22;246(4):513–526. doi: 10.1002/cne.902460408. [DOI] [PubMed] [Google Scholar]
- Baker D. G. Parasympathetic motor pathways to the trachea: recent morphologic and electrophysiologic studies. Clin Chest Med. 1986 Jun;7(2):223–229. [PubMed] [Google Scholar]
- Bałuk P., Fujiwara T., Matsuda S. The fine structure of the ganglia of the guinea-pig trachea. Cell Tissue Res. 1985;239(1):51–60. doi: 10.1007/BF00214902. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Adams P. R. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature. 1980 Feb 14;283(5748):673–676. doi: 10.1038/283673a0. [DOI] [PubMed] [Google Scholar]
- Brown D. A. M currents. Ion Channels. 1988;1:55–94. doi: 10.1007/978-1-4615-7302-9_2. [DOI] [PubMed] [Google Scholar]
- Brown H., Difrancesco D. Voltage-clamp investigations of membrane currents underlying pace-maker activity in rabbit sino-atrial node. J Physiol. 1980 Nov;308:331–351. doi: 10.1113/jphysiol.1980.sp013474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron A. R., Coburn R. F. Electrical and anatomic characteristics of cells of ferret paratracheal ganglion. Am J Physiol. 1984 May;246(5 Pt 1):C450–C458. doi: 10.1152/ajpcell.1984.246.5.C450. [DOI] [PubMed] [Google Scholar]
- Chiang C. H., Gabella G. Quantitative study of the ganglion neurons of the mouse trachea. Cell Tissue Res. 1986;246(2):243–252. doi: 10.1007/BF00215886. [DOI] [PubMed] [Google Scholar]
- Coburn R. F., Kalia M. P. Morphological features of spiking and nonspiking cells in the paratracheal ganglion of the ferret. J Comp Neurol. 1986 Dec 15;254(3):341–351. doi: 10.1002/cne.902540307. [DOI] [PubMed] [Google Scholar]
- Coburn R. F., Tomita T. Evidence for nonadrenergic inhibitory nerves in the guinea pig trachealis muscle. Am J Physiol. 1973 May;224(5):1072–1080. doi: 10.1152/ajplegacy.1973.224.5.1072. [DOI] [PubMed] [Google Scholar]
- Coleman R. A., Levy G. P. A non-adrenergic inhibitory nervous pathway in guinea-pig trachea. Br J Pharmacol. 1974 Oct;52(2):167–174. doi: 10.1111/j.1476-5381.1974.tb09697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constanti A., Galvan M. Fast inward-rectifying current accounts for anomalous rectification in olfactory cortex neurones. J Physiol. 1983 Feb;335:153–178. doi: 10.1113/jphysiol.1983.sp014526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crepel F., Penit-Soria J. Inward rectification and low threshold calcium conductance in rat cerebellar Purkinje cells. An in vitro study. J Physiol. 1986 Mar;372:1–23. doi: 10.1113/jphysiol.1986.sp015993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey R. D., Shannon W. A., Jr, Said S. I. Localization of VIP-immunoreactive nerves in airways and pulmonary vessels of dogs, cat, and human subjects. Cell Tissue Res. 1981;220(2):231–238. doi: 10.1007/BF00210505. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D., Ojeda C. Properties of the current if in the sino-atrial node of the rabbit compared with those of the current iK, in Purkinje fibres. J Physiol. 1980 Nov;308:353–367. doi: 10.1113/jphysiol.1980.sp013475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond L., O'Donnell M. A nonadrenergic vagal inhibitory pathway to feline airways. Science. 1980 Apr 11;208(4440):185–188. doi: 10.1126/science.7361114. [DOI] [PubMed] [Google Scholar]
- FISHER A. W. THE INTRINSIC INNERVATION OF THE TRACHEA. J Anat. 1964 Jan;98:117–124. [PMC free article] [PubMed] [Google Scholar]
- Fowler J. C., Greene R., Weinreich D. Two calcium-sensitive spike after-hyperpolarizations in visceral sensory neurones of the rabbit. J Physiol. 1985 Aug;365:59–75. doi: 10.1113/jphysiol.1985.sp015759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay L. A., Stanfield P. R. Cs(+) causes a voltage-dependent block of inward K currents in resting skeletal muscle fibres. Nature. 1977 May 12;267(5607):169–170. doi: 10.1038/267169a0. [DOI] [PubMed] [Google Scholar]
- HONJIN R. On the nerve supply of the lung of the mouse, with special reference to the structure of the peripheral vegetative nervous system. J Comp Neurol. 1956 Oct;105(3):587–625. doi: 10.1002/cne.901050308. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Miyazaki S., Moody W., Patlak J. Blocking effects of barium and hydrogen ions on the potassium current during anomalous rectification in the starfish egg. J Physiol. 1978 Jun;279:167–185. doi: 10.1113/jphysiol.1978.sp012338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Miyazaki S., Rosenthal N. P. Potassium current and the effect of cesium on this current during anomalous rectification of the egg cell membrane of a starfish. J Gen Physiol. 1976 Jun;67(6):621–638. doi: 10.1085/jgp.67.6.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell J. V., Adams P. R. Voltage-clamp analysis of muscarinic excitation in hippocampal neurons. Brain Res. 1982 Oct 28;250(1):71–92. doi: 10.1016/0006-8993(82)90954-4. [DOI] [PubMed] [Google Scholar]
- Lundberg J. M., Saria A. Polypeptide-containing neurons in airway smooth muscle. Annu Rev Physiol. 1987;49:557–572. doi: 10.1146/annurev.ph.49.030187.003013. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. A voltage-clamp analysis of inward (anomalous) rectification in mouse spinal sensory ganglion neurones. J Physiol. 1983 Jul;340:19–45. doi: 10.1113/jphysiol.1983.sp014747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell R. A., Herbert D. A., Baker D. G., Basbaum C. B. In vivo activity of tracheal parasympathetic ganglion cells innervating tracheal smooth muscle. Brain Res. 1987 Dec 22;437(1):157–160. doi: 10.1016/0006-8993(87)91537-x. [DOI] [PubMed] [Google Scholar]
- Morita K., North R. A., Tokimasa T. The calcium-activated potassium conductance in guinea-pig myenteric neurones. J Physiol. 1982 Aug;329:341–354. doi: 10.1113/jphysiol.1982.sp014306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi S., North R. A. Intracellular recording from the myenteric plexus of the guinea-pig ileum. J Physiol. 1973 Jun;231(3):471–491. doi: 10.1113/jphysiol.1973.sp010244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pack R. J., Al-Ugaily L. H., Widdicombe J. G. The innervation of the trachea and extrapulmonary bronchi of the mouse. Cell Tissue Res. 1984;238(1):61–68. doi: 10.1007/BF00215145. [DOI] [PubMed] [Google Scholar]
- Richardson J. B., Bouchard T. Demonstration of a nonadrenergic inhibitory nervous system in the trachea of the guinea pig. J Allergy Clin Immunol. 1975 Dec;56(6):473–480. doi: 10.1016/0091-6749(75)90065-2. [DOI] [PubMed] [Google Scholar]
- Richardson J., Béland J. Nonadrenergic inhibitory nervous system in human airways. J Appl Physiol. 1976 Nov;41(5 Pt 1):764–771. doi: 10.1152/jappl.1976.41.5.764. [DOI] [PubMed] [Google Scholar]
- Standen N. B., Stanfield P. R. A potential- and time-dependent blockade of inward rectification in frog skeletal muscle fibres by barium and strontium ions. J Physiol. 1978 Jul;280:169–191. doi: 10.1113/jphysiol.1978.sp012379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddman R., Alumets J., Densert O., Håkanson R., Sundler F. Occurrence and distribution of VIP nerves in the nasal mucosa and tracheobronchial wall. Acta Otolaryngol. 1978 Nov-Dec;86(5-6):443–448. doi: 10.3109/00016487809107524. [DOI] [PubMed] [Google Scholar]
- Yip P., Palombini B., Coburn R. F. Inhibitory innervation to the guinea pig trachealis muscle. J Appl Physiol Respir Environ Exerc Physiol. 1981 Feb;50(2):374–382. doi: 10.1152/jappl.1981.50.2.374. [DOI] [PubMed] [Google Scholar]


