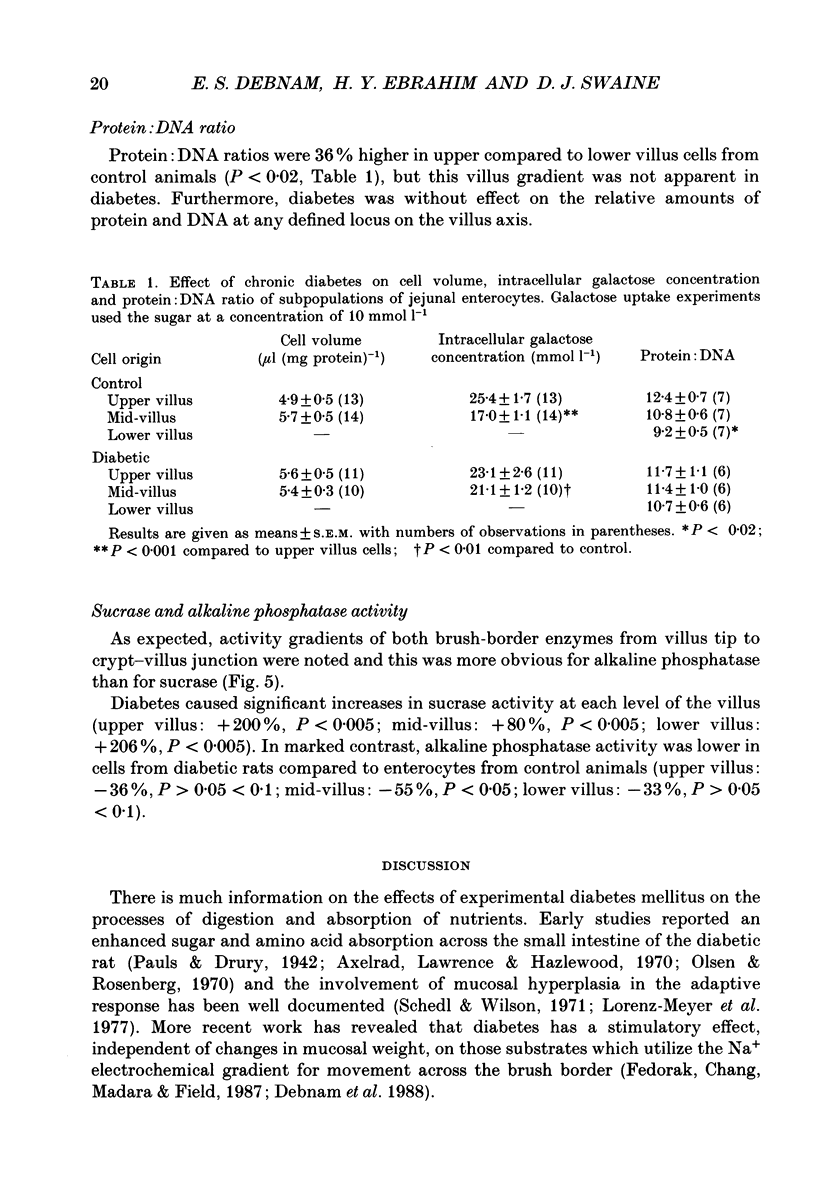
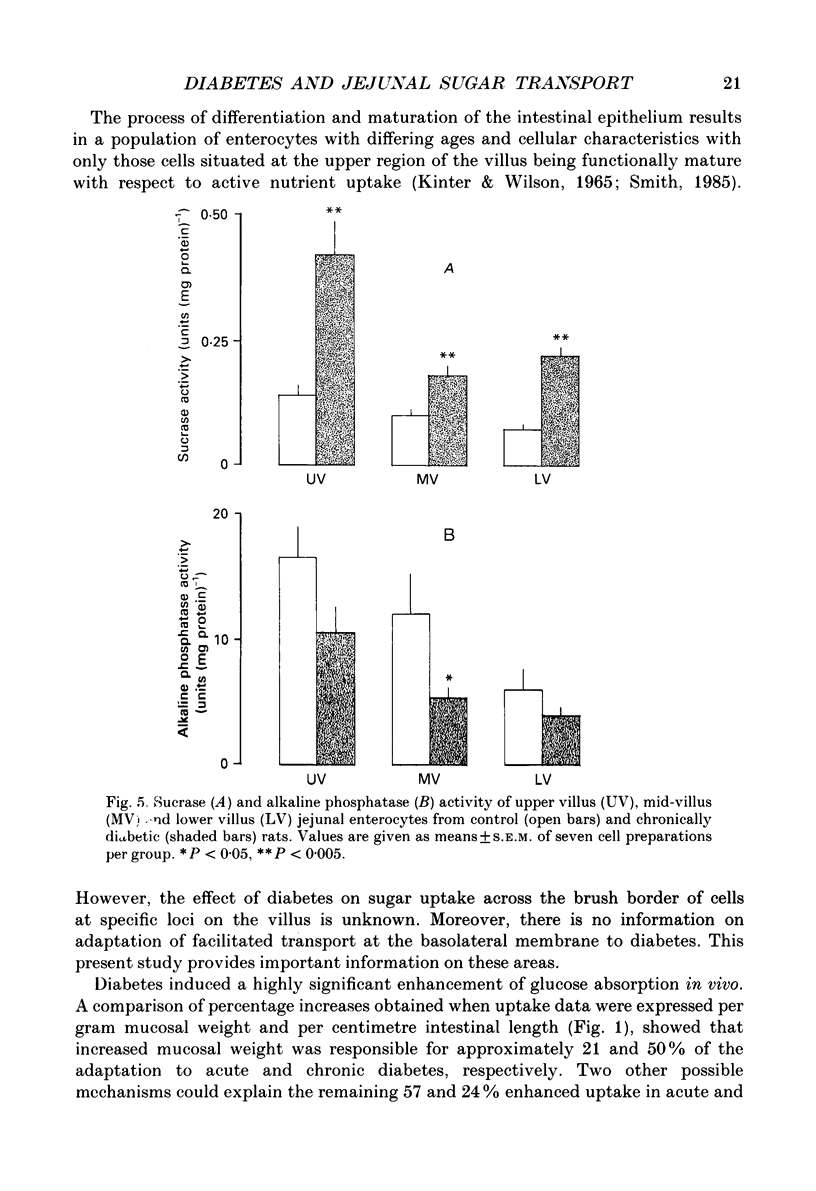
Abstract
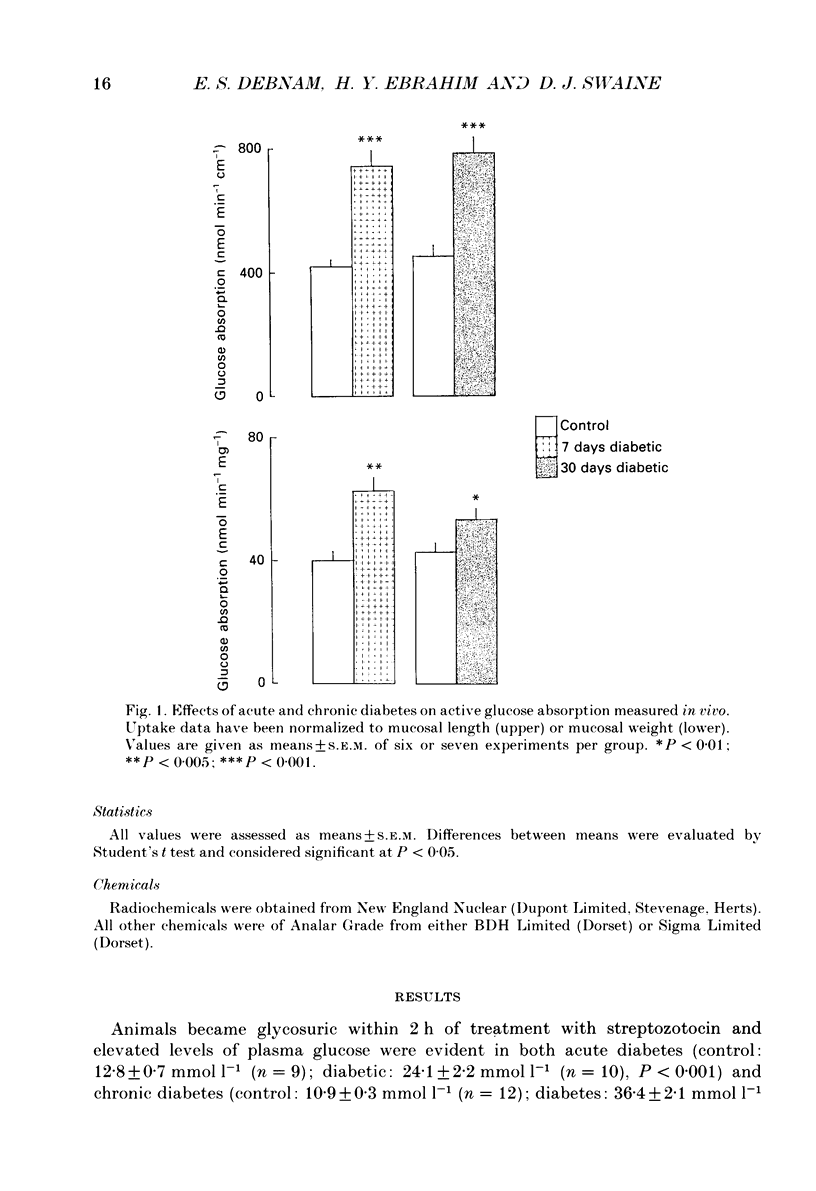
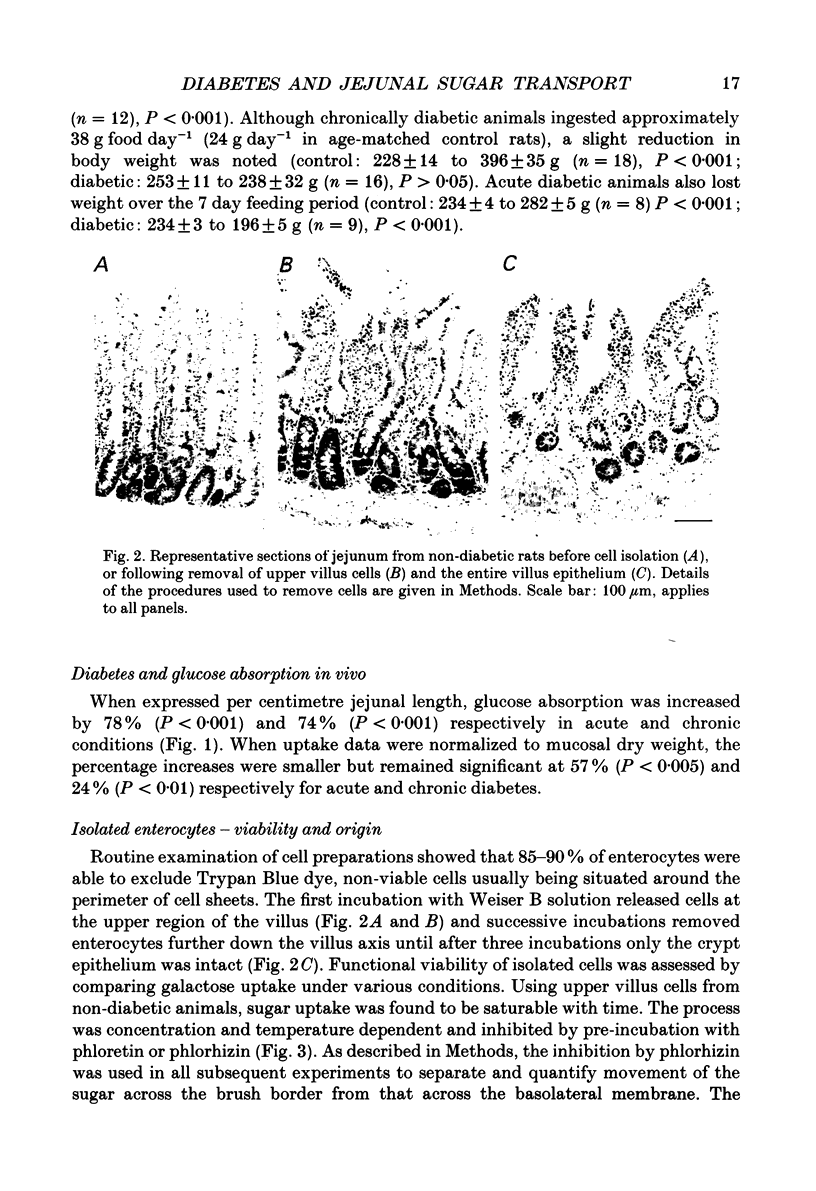
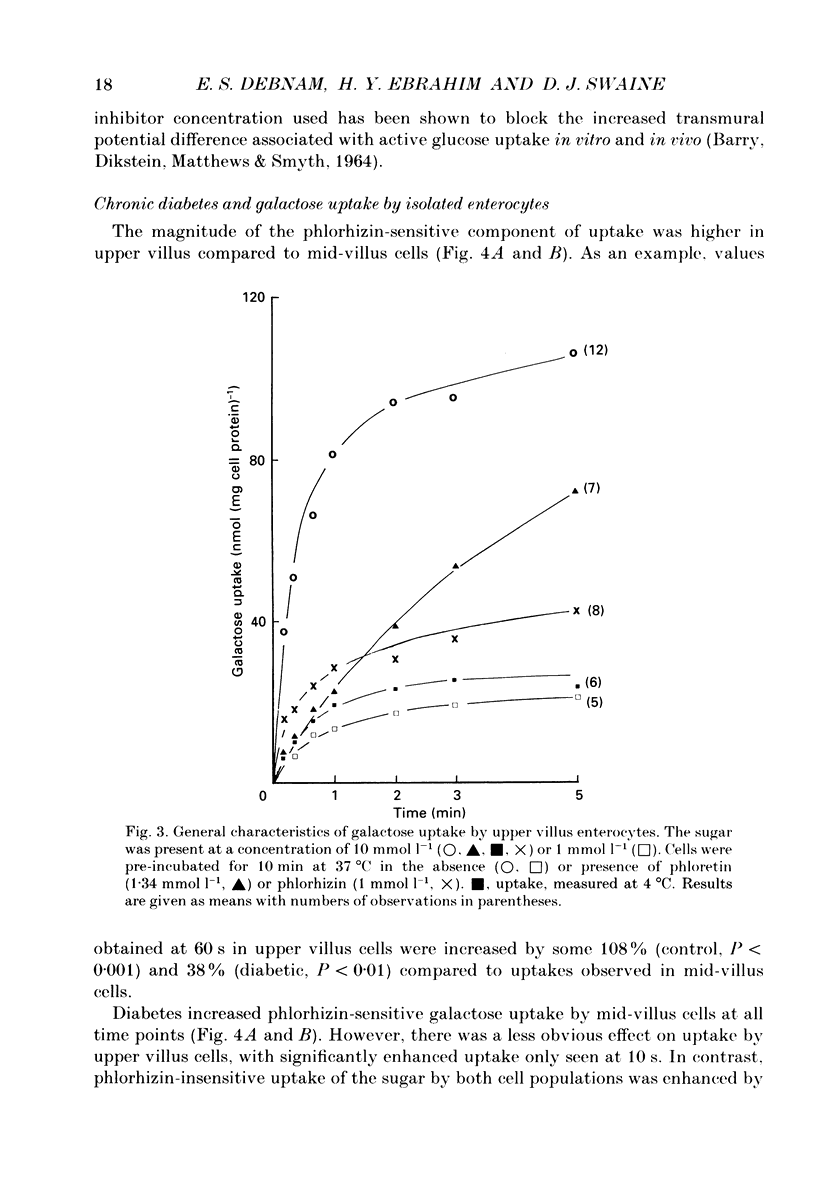
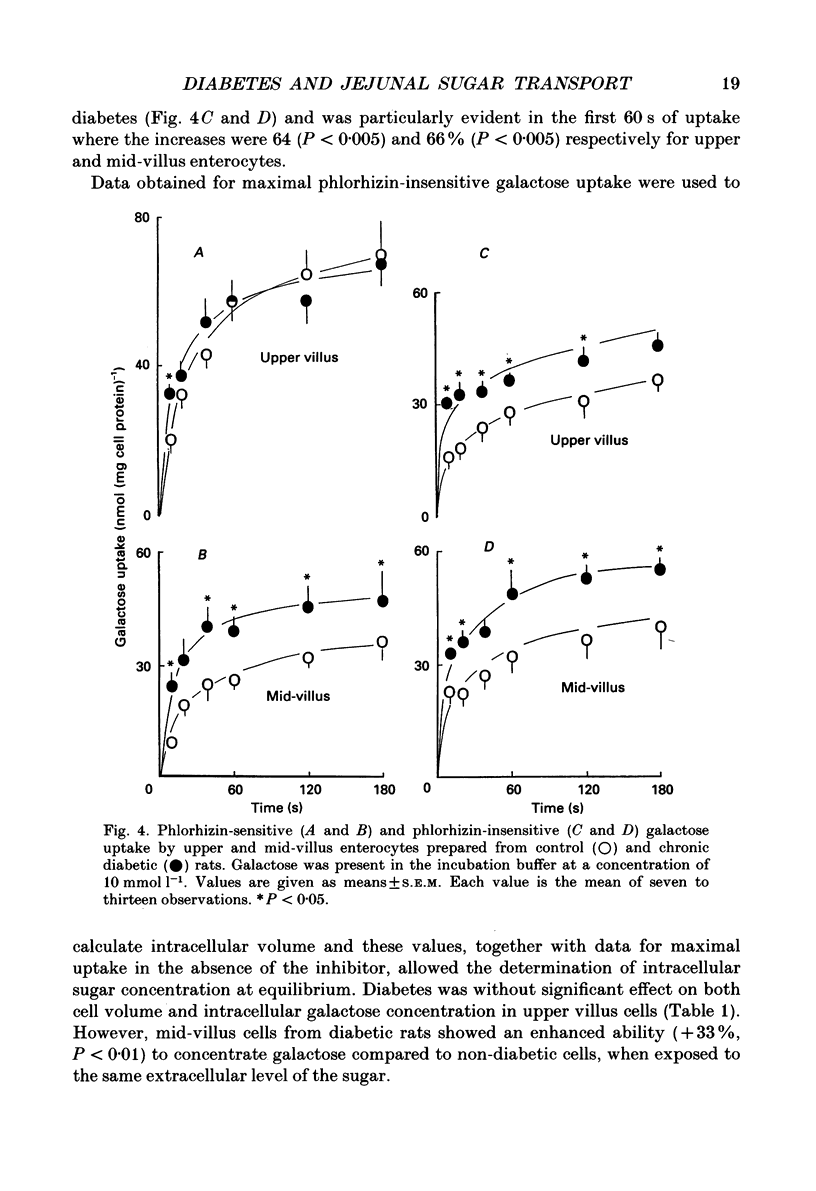
1. The effects of streptozotocin-induced diabetes mellitus on active jejunal glucose uptake in vivo, and on galactose movement across the brush-border (phlorhizin-sensitive) and basolateral (phlorhizin-insensitive) membranes of isolated upper and mid-villus enterocytes has been studied. 2. Chronic diabetes increased unidirectional phlorhizin-sensitive galactose uptake by mid-villus but not upper villus cells. In contrast, phlorhizin-insensitive uptake by both cell populations was enhanced by diabetes. 3. Diabetes increased glucose absorption in vivo by mechanisms which were unrelated to hyperphagia. Mucosal hyperplasia acting together with an epithelium containing a higher proportion of mature enterocytes is the most likely explanation for the response. 4. We conclude that, during diabetes, the mid-villus region is an important site of adaptation with functional changes occurring at both the brush-border and basolateral membranes. The increased hexose transport ability of the basolateral membrane is retained during cell transit along the villus.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrad A. D., Lawrence A. L., Hazelwood R. L. Fasting and alloxan diabetes effects of intestinal transport of monosaccharides. Am J Physiol. 1970 Oct;219(4):860–864. doi: 10.1152/ajplegacy.1970.219.4.860. [DOI] [PubMed] [Google Scholar]
- BARRY R. J., DIKSTEIN S., MATTHEWS J., SMYTH D. H., WRIGHT E. M. ELECTRICAL POTENTIALS ASSOCIATED WITH INTESTINAL SUGAR TRANSFER. J Physiol. 1964 Jun;171:316–338. doi: 10.1113/jphysiol.1964.sp007379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Csáky T. Z., Fischer E. Intestinal sugar transport in experimental diabetes. Diabetes. 1981 Jul;30(7):568–574. doi: 10.2337/diab.30.7.568. [DOI] [PubMed] [Google Scholar]
- DAHLQVIST A. METHOD FOR ASSAY OF INTESTINAL DISACCHARIDASES. Anal Biochem. 1964 Jan;7:18–25. doi: 10.1016/0003-2697(64)90115-0. [DOI] [PubMed] [Google Scholar]
- Debnam E. S., Ebrahim H. Y. Diabetes mellitus and the sodium electrochemical gradient across the brush border membrane of rat intestinal enterocytes. J Endocrinol. 1989 Dec;123(3):453–459. doi: 10.1677/joe.0.1230453. [DOI] [PubMed] [Google Scholar]
- Debnam E. S., Karasov W. H., Thompson C. S. Nutrient uptake by rat enterocytes during diabetes mellitus; evidence for an increased sodium electrochemical gradient. J Physiol. 1988 Mar;397:503–512. doi: 10.1113/jphysiol.1988.sp017015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnam E. S., Levin R. J. An experimental method of identifying and quantifying the active transfer electrogenic component from the diffusive component during sugar absorption measured in vivo. J Physiol. 1975 Mar;246(1):181–196. doi: 10.1113/jphysiol.1975.sp010885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnam E. S., Thompson C. S. The effect of fasting on the potential difference across the brush-border membrane of enterocytes in rat small intestine. J Physiol. 1984 Oct;355:449–456. doi: 10.1113/jphysiol.1984.sp015430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorak R. N., Chang E. B., Madara J. L., Field M. Intestinal adaptation to diabetes. Altered Na-dependent nutrient absorption in streptozocin-treated chronically diabetic rats. J Clin Invest. 1987 Jun;79(6):1571–1578. doi: 10.1172/JCI112991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorak R. N., Gershon M. D., Field M. Induction of intestinal glucose carriers in streptozocin-treated chronically diabetic rats. Gastroenterology. 1989 Jan;96(1):37–44. doi: 10.1016/0016-5085(89)90761-0. [DOI] [PubMed] [Google Scholar]
- Forstner G. G., Sabesin S. M., Isselbacher K. J. Rat intestinal microvillus membranes. Purification and biochemical characterization. Biochem J. 1968 Jan;106(2):381–390. doi: 10.1042/bj1060381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman H. J., Johnston G., Quamme G. A. Sodium-dependent D-glucose transport in brush-border membrane vesicles from isolated rat small intestinal villus and crypt epithelial cells. Can J Physiol Pharmacol. 1987 Jun;65(6):1213–1219. doi: 10.1139/y87-191. [DOI] [PubMed] [Google Scholar]
- Harig J. M., Barry J. A., Rajendran V. M., Soergel K. H., Ramaswamy K. D-glucose and L-leucine transport by human intestinal brush-border membrane vesicles. Am J Physiol. 1989 Mar;256(3 Pt 1):G618–G623. doi: 10.1152/ajpgi.1989.256.3.G618. [DOI] [PubMed] [Google Scholar]
- Hopfer U., Sigrist-Nelson K., Ammann E., Murer H. Differences in neutral amino acid and glucose transport between brush border and basolateral plasma membrane of intestinal epithelial cells. J Cell Physiol. 1976 Dec;89(4):805–810. doi: 10.1002/jcp.1040890447. [DOI] [PubMed] [Google Scholar]
- Karasov W. H., Diamond J. M. Adaptive regulation of sugar and amino acid transport by vertebrate intestine. Am J Physiol. 1983 Oct;245(4):G443–G462. doi: 10.1152/ajpgi.1983.245.4.G443. [DOI] [PubMed] [Google Scholar]
- Kimmich G. A., Randles J. 2-Deoxyglucose transport by intestinal epithelial cells isolated from the chick. J Membr Biol. 1976 Jun 30;27(4):363–379. doi: 10.1007/BF01869146. [DOI] [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Lorenz-Meyer H., Thiel F., Menge H., Gottesbüren H., Riecken E. O. Structural and functional studies on the transformation of the intestinal mucosa in rats with experimental diabetes. Res Exp Med (Berl) 1977 Jun 29;170(2):89–99. doi: 10.1007/BF01851379. [DOI] [PubMed] [Google Scholar]
- Maenz D. D., Cheeseman C. I. Effect of hyperglycemia on D-glucose transport across the brush-border and basolateral membrane of rat small intestine. Biochim Biophys Acta. 1986 Aug 21;860(2):277–285. doi: 10.1016/0005-2736(86)90524-9. [DOI] [PubMed] [Google Scholar]
- Olsen W. A., Rosenberg I. H. Intestinal transport of sugars and amino acids in diabetic rats. J Clin Invest. 1970 Jan;49(1):96–105. doi: 10.1172/JCI106227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randles J., Kimmich G. A. Effects of phloretin and theophylline on 3-O-methylglucose transport by intestinal epithelial cells. Am J Physiol. 1978 Mar;234(3):C64–C72. doi: 10.1152/ajpcell.1978.234.3.C64. [DOI] [PubMed] [Google Scholar]
- Schedl H. P., Wilson H. D. Effects of diabetes on intestinal growth and hexose transport in the rat. Am J Physiol. 1971 Jun;220(6):1739–1745. doi: 10.1152/ajplegacy.1971.220.6.1739. [DOI] [PubMed] [Google Scholar]
- Weiser M. M. Intestinal epithelial cell surface membrane glycoprotein synthesis. I. An indicator of cellular differentiation. J Biol Chem. 1973 Apr 10;248(7):2536–2541. [PubMed] [Google Scholar]



