Abstract
1. Intramembrane charge movement and Ca2+ currents were monitored in voltage-clamped segments of frog skeletal muscle fibres using the triple-Vaseline-gap technique. Calcium signals were measured in current-clamped fibres using either of the indicators Arsenazo III or Antipyrylazo III. 2. Non-linear capacitative currents (charge 1) were obtained using a subtraction procedure which employed either a -20 mV control pulse from a holding potential of -100 mV or alternatively a control pulse to +80 mV in depolarized fibres. The amount of charge mobilized depended on voltage according to a two-state Boltzmann function. The total charge (Qmax) was increased by ca 100% and the steepness parameter (k) by ca 70% when a +80 mV control pulse was used. 3. Thiocyanate (SCN-) and other lyotropic anions reversibly shifted the voltage dependence of mobilized charge towards negative potentials. Qmax was not significantly affected. 'Off' charge tails were greatly prolonged by lyotropic anions. 4. Extracellularly applied lyotropic anions affected the dihydropyridine-sensitive Ca2+ current by shifting the I-V relation toward more negative voltages and delaying deactivation of the tail currents. 5. The effects of lyotropic anions did not depend on whether the anion was introduced intracellularly or extracellularly. 6. Extracellular SCN- reversibly increased the peak amplitude and rate of rise of Ca signals, and decreased the latent period between stimulation and onset of the Ca signal. 7. It is concluded that lyotropic anions have similar effects on Ca2+ currents and on charge movement.
Full text
PDF



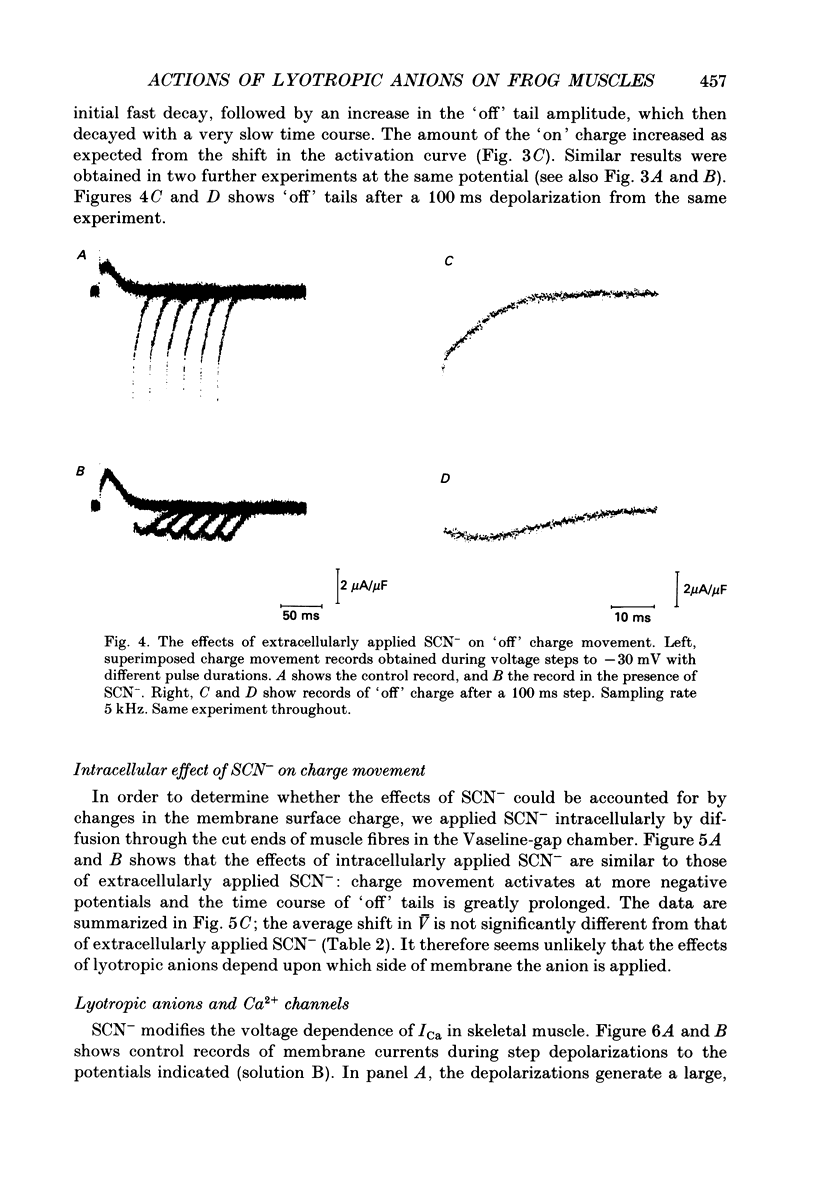
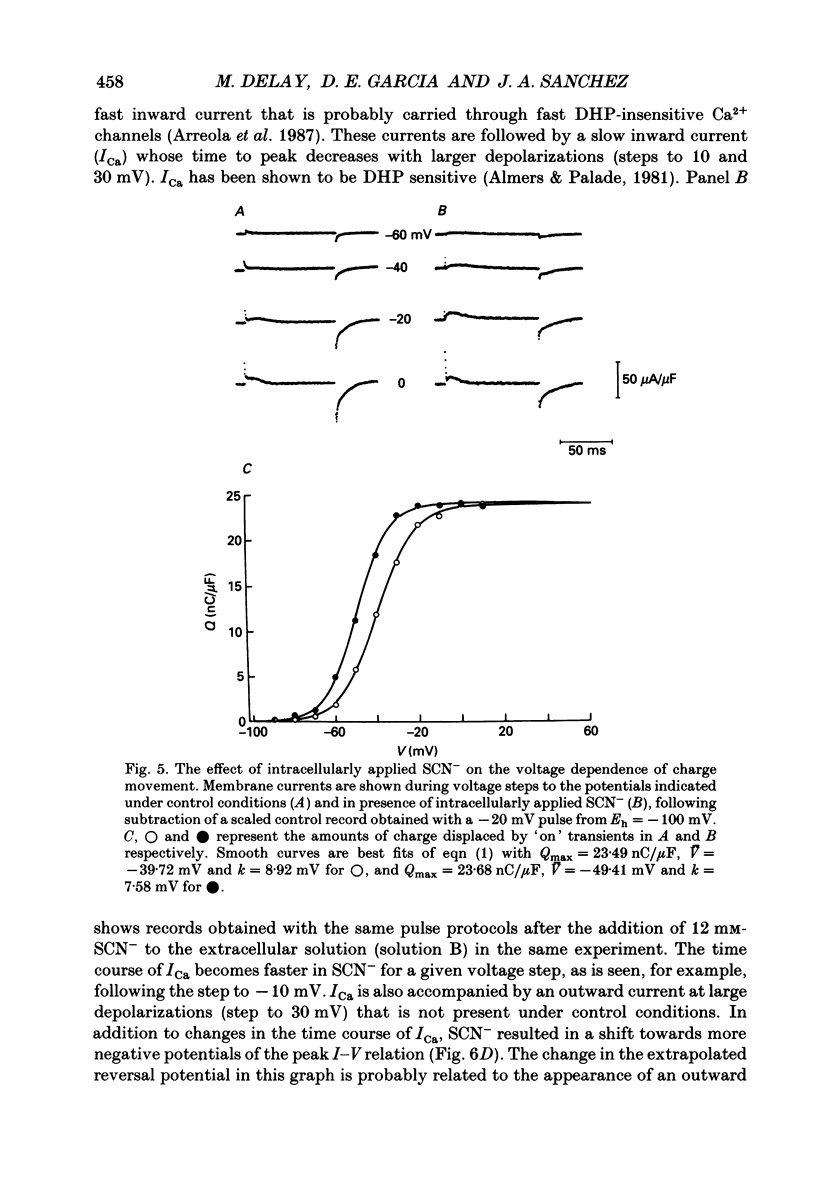

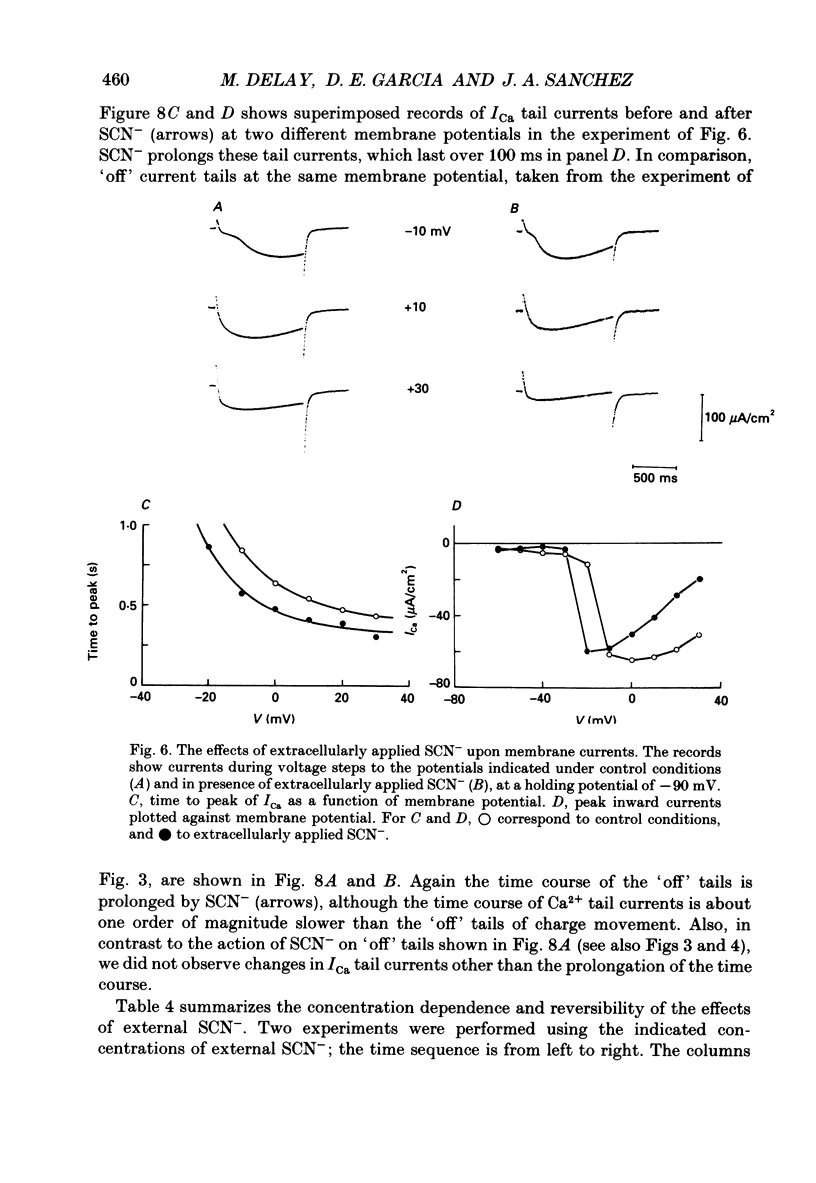
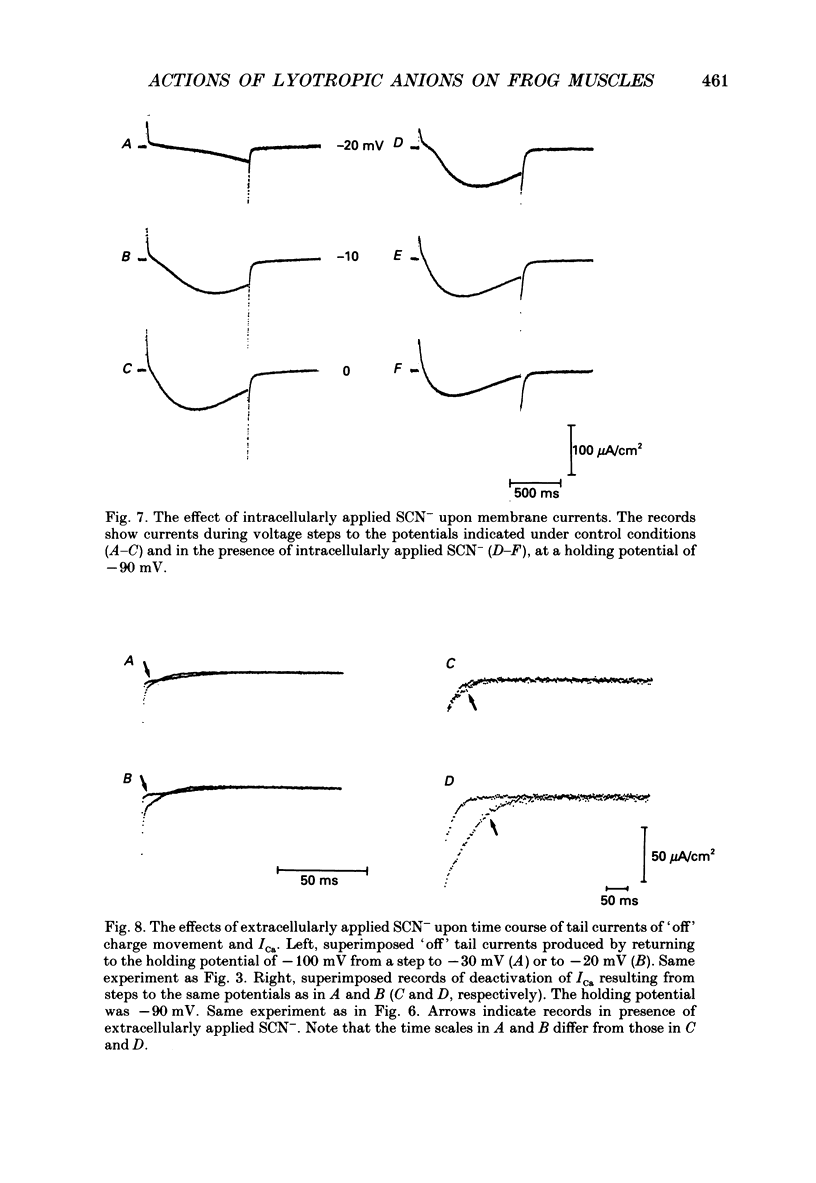
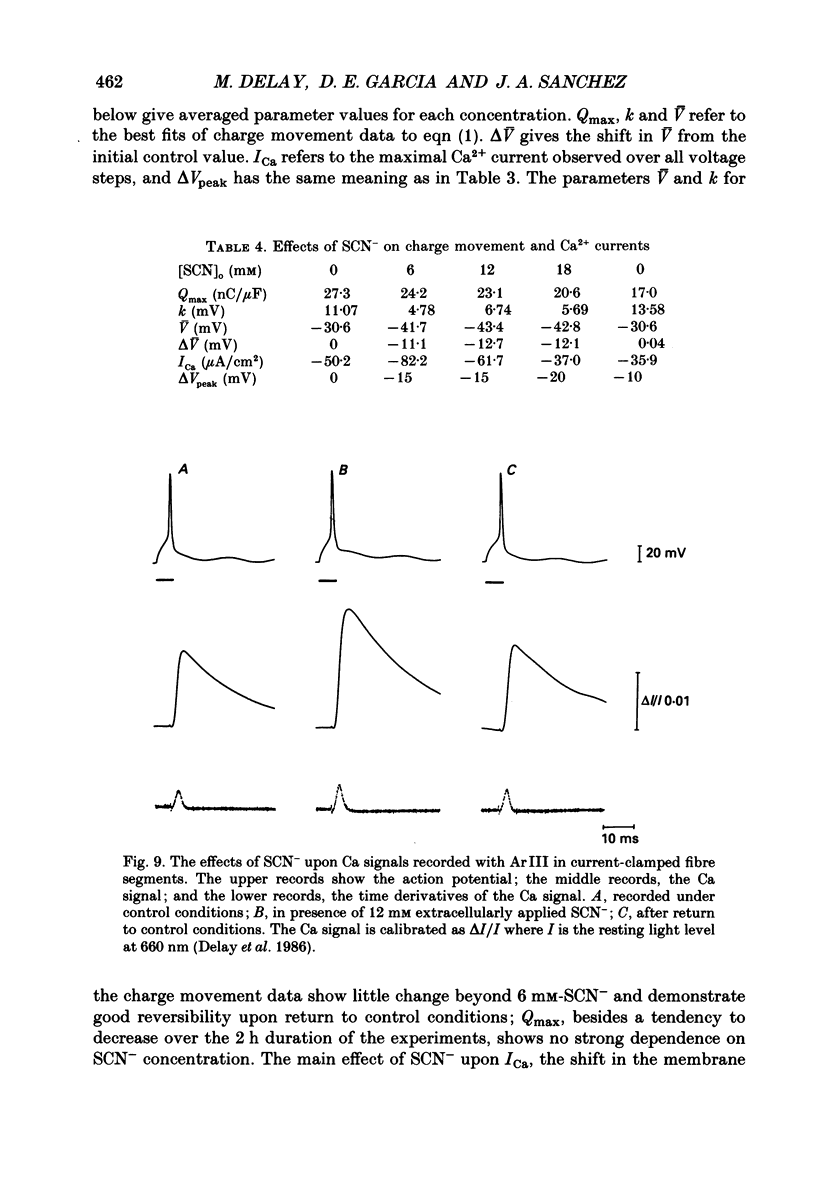
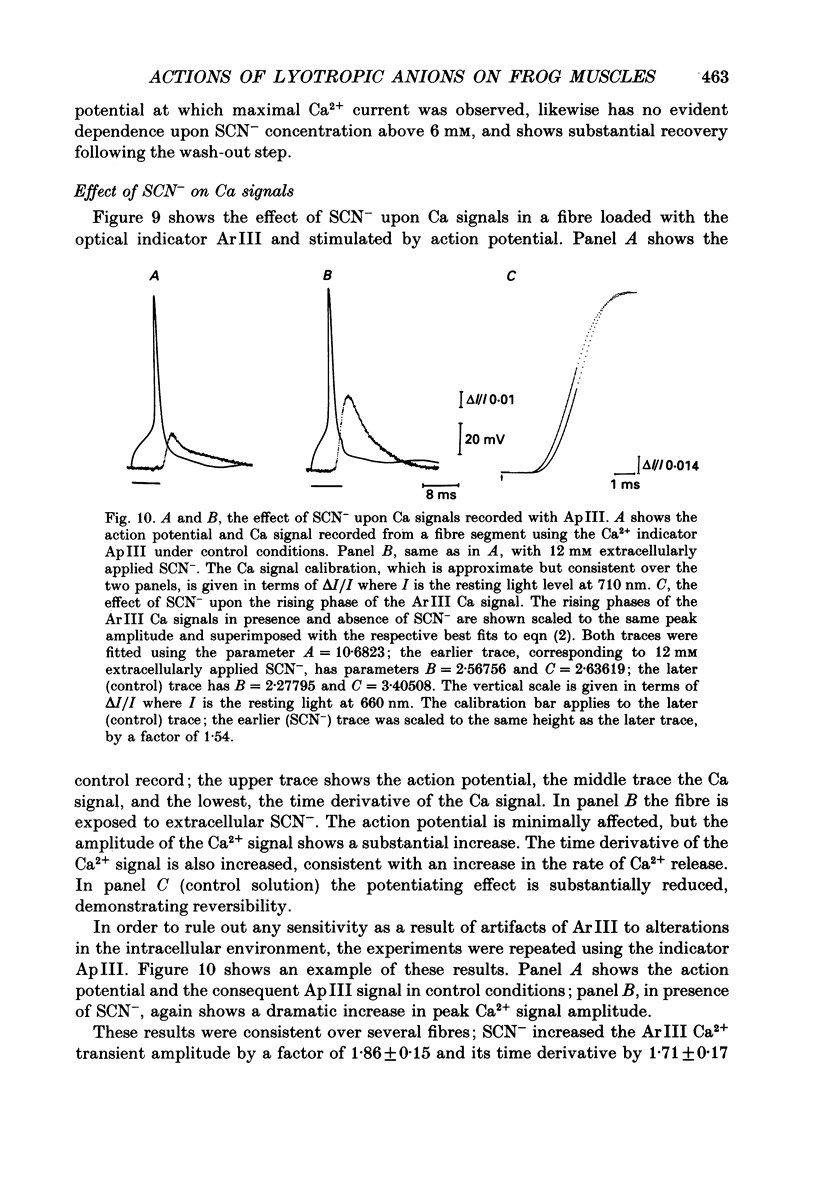






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W. Gating currents and charge movements in excitable membranes. Rev Physiol Biochem Pharmacol. 1978;82:96–190. doi: 10.1007/BFb0030498. [DOI] [PubMed] [Google Scholar]
- Almers W., McCleskey E. W., Palade P. T. A non-selective cation conductance in frog muscle membrane blocked by micromolar external calcium ions. J Physiol. 1984 Aug;353:565–583. doi: 10.1113/jphysiol.1984.sp015351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almers W., Palade P. T. Slow calcium and potassium currents across frog muscle membrane: measurements with a vaseline-gap technique. J Physiol. 1981 Mar;312:159–176. doi: 10.1113/jphysiol.1981.sp013622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M. Sodium channels and gating currents. Physiol Rev. 1981 Jul;61(3):644–683. doi: 10.1152/physrev.1981.61.3.644. [DOI] [PubMed] [Google Scholar]
- Arreola J., Calvo J., García M. C., Sánchez J. A. Modulation of calcium channels of twitch skeletal muscle fibres of the frog by adrenaline and cyclic adenosine monophosphate. J Physiol. 1987 Dec;393:307–330. doi: 10.1113/jphysiol.1987.sp016825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beam K. G., Knudson C. M., Powell J. A. A lethal mutation in mice eliminates the slow calcium current in skeletal muscle cells. Nature. 1986 Mar 13;320(6058):168–170. doi: 10.1038/320168a0. [DOI] [PubMed] [Google Scholar]
- Brum G., Fitts R., Pizarro G., Ríos E. Voltage sensors of the frog skeletal muscle membrane require calcium to function in excitation-contraction coupling. J Physiol. 1988 Apr;398:475–505. doi: 10.1113/jphysiol.1988.sp017053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brum G., Rios E. Intramembrane charge movement in frog skeletal muscle fibres. Properties of charge 2. J Physiol. 1987 Jun;387:489–517. doi: 10.1113/jphysiol.1987.sp016586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Rakowski R. F., Schneider M. F. A non-linear voltage dependent charge movement in frog skeletal muscle. J Physiol. 1976 Jan;254(2):245–283. doi: 10.1113/jphysiol.1976.sp011232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csernoch L., Kovács L., Szücs G. Perchlorate and the relationship between charge movement and contractile activation in frog skeletal muscle fibres. J Physiol. 1987 Sep;390:213–227. doi: 10.1113/jphysiol.1987.sp016695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani J. A., Sanchez J. A., Hille B. Lyotropic anions. Na channel gating and Ca electrode response. J Gen Physiol. 1983 Feb;81(2):255–281. doi: 10.1085/jgp.81.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delay M., Ribalet B., Vergara J. Caffeine potentiation of calcium release in frog skeletal muscle fibres. J Physiol. 1986 Jun;375:535–559. doi: 10.1113/jphysiol.1986.sp016132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulhunty A. F., Gage P. W. Effects of extracellular calcium concentration and dihydropyridines on contraction in mammalian skeletal muscle. J Physiol. 1988 May;399:63–80. doi: 10.1113/jphysiol.1988.sp017068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomolla M., Gottschalk G., Lüttgau H. C. Perchlorate-induced alterations in electrical and mechanical parameters of frog skeletal muscle fibres. J Physiol. 1983 Oct;343:197–214. doi: 10.1113/jphysiol.1983.sp014888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The effect of nitrate and other anions on the mechanical response of single muscle fibres. J Physiol. 1960 Sep;153:404–412. doi: 10.1113/jphysiol.1960.sp006542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOROWICZ P. THE EFFECTS OF ANIONS ON EXCITABLE CELLS. Pharmacol Rev. 1964 Jun;16:193–221. [PubMed] [Google Scholar]
- Hille B., Campbell D. T. An improved vaseline gap voltage clamp for skeletal muscle fibers. J Gen Physiol. 1976 Mar;67(3):265–293. doi: 10.1085/jgp.67.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B., Woodhull A. M., Shapiro B. I. Negative surface charge near sodium channels of nerve: divalent ions, monovalent ions, and pH. Philos Trans R Soc Lond B Biol Sci. 1975 Jun 10;270(908):301–318. doi: 10.1098/rstb.1975.0011. [DOI] [PubMed] [Google Scholar]
- Horowicz P., Schneider M. F. Membrane charge movement in contracting and non-contracting skeletal muscle fibres. J Physiol. 1981 May;314:565–593. doi: 10.1113/jphysiol.1981.sp013725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. L. 'Off' tails of intramembrane charge movements in frog skeletal muscle in perchlorate-containing solutions. J Physiol. 1987 Mar;384:491–509. doi: 10.1113/jphysiol.1987.sp016466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. L. Intramembrane charge movements in skeletal muscle. Physiol Rev. 1988 Oct;68(4):1197–1147. doi: 10.1152/physrev.1988.68.4.1197. [DOI] [PubMed] [Google Scholar]
- Huang C. L. The differential effects of twitch potentiators on charge movements in frog skeletal muscle. J Physiol. 1986 Nov;380:17–33. doi: 10.1113/jphysiol.1986.sp016269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao C. Y., Stanfield P. R. Actions of some anions on electrical properties and mechanical threshold of frog twitch muscle. J Physiol. 1968 Sep;198(2):291–309. doi: 10.1113/jphysiol.1968.sp008607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb G. D., Walsh T. Calcium currents, charge movement and dihydropyridine binding in fast- and slow-twitch muscles of rat and rabbit. J Physiol. 1987 Dec;393:595–617. doi: 10.1113/jphysiol.1987.sp016843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCleskey E. W. Calcium channels and intracellular calcium release are pharmacologically different in frog skeletal muscle. J Physiol. 1985 Apr;361:231–249. doi: 10.1113/jphysiol.1985.sp015643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieger F., Bournaud R., Shimahara T., Garcia L., Pinçon-Raymond M., Romey G., Lazdunski M. Restoration of dysgenic muscle contraction and calcium channel function by co-culture with normal spinal cord neurons. Nature. 1987 Dec 10;330(6148):563–566. doi: 10.1038/330563a0. [DOI] [PubMed] [Google Scholar]
- Rios E., Brum G. Involvement of dihydropyridine receptors in excitation-contraction coupling in skeletal muscle. Nature. 1987 Feb 19;325(6106):717–720. doi: 10.1038/325717a0. [DOI] [PubMed] [Google Scholar]
- Sanchez J. A., Stefani E. Inward calcium current in twitch muscle fibres of the frog. J Physiol. 1978 Oct;283:197–209. doi: 10.1113/jphysiol.1978.sp012496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanguinetti M. C., Kass R. S. Voltage-dependent block of calcium channel current in the calf cardiac Purkinje fiber by dihydropyridine calcium channel antagonists. Circ Res. 1984 Sep;55(3):336–348. doi: 10.1161/01.res.55.3.336. [DOI] [PubMed] [Google Scholar]
- Schneider M. F., Chandler W. K. Voltage dependent charge movement of skeletal muscle: a possible step in excitation-contraction coupling. Nature. 1973 Mar 23;242(5395):244–246. doi: 10.1038/242244a0. [DOI] [PubMed] [Google Scholar]
- Sánchez J. A., Stefani E. Kinetic properties of calcium channels of twitch muscle fibres of the frog. J Physiol. 1983 Apr;337:1–17. doi: 10.1113/jphysiol.1983.sp014607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe T., Beam K. G., Powell J. A., Numa S. Restoration of excitation-contraction coupling and slow calcium current in dysgenic muscle by dihydropyridine receptor complementary DNA. Nature. 1988 Nov 10;336(6195):134–139. doi: 10.1038/336134a0. [DOI] [PubMed] [Google Scholar]
- Vergara J., Caputo C. Effects of tetracaine on charge movements and calcium signals in frog skeletal muscle fibers. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1477–1481. doi: 10.1073/pnas.80.5.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woll K. H., Leibowitz M. D., Neumcke B., Hille B. A high-conductance anion channel in adult amphibian skeletal muscle. Pflugers Arch. 1987 Dec;410(6):632–640. doi: 10.1007/BF00581324. [DOI] [PubMed] [Google Scholar]


