Abstract
1. Synergistic activation of a GTP-binding protein (G protein) by external serotonin (5-hydroxytryptamine, 5-HT) and internally applied guanosine-5'-O-(3-thiotriphosphate (GTP gamma S) in hamster eggs was demonstrated by the facilitation of repetitive increases in cytoplasmic Ca2+ as measured by their associated hyperpolarizing responses (HRs) and by aequorin luminescence. 2. Rapid application of 70 nM-5-HT caused a single HR of 10-12 s duration and with a delay of 80 s. The critical concentration of 5-HT to cause an HR was 50 nM. 3. With 10 microM-5-HT four to six HRs were often elicited with a delay to the first HR of 8-30 s. HRs disappeared after prolonged or repeated application of 5-HT, indicating an apparent desensitization. 4. 5-HT-induced HRs were completely inhibited by the protein kinase C (PKC) activator phorbol 12-myristate 13-acetate (TPA) (100 nM). Conversely, the PKC inhibitor sphingosine (2 microM) enhanced the series of HRs by shortening the delay to the first HR (3-9 s) and by causing more HRs. 5. Ionophoretic injection of GTP gamma S into the egg usually produced a large HR with a delay of 120-240 s followed by a series of much smaller HRs. When 5-HT was applied within 1 min of injection of GTP gamma S. 70 nM-5-HT induced a number of large HRs and even 1 nM-5-HT could induce HR(s). In contrast, when 5-HT was applied after the size of GTP gamma S-induced HRs had declined, as much as 10 microM-5-HT could only elicit a single large HR. Thus, GTP gamma S apparently caused a sensitization and then a desensitization of the action of 5-HT. 6. GTP gamma S-induced Ca2+ transients were facilitated when injected in the presence of 5-HT concentrations as low as 0.1 nM. The time delay to the first HR was 65 s in 0.1 nM-5-HT or 4 s in 100 nM-5-HT whereas it was 170 s without 5-HT (mean values). The magnitude as well as frequency of HRs succeeding the first HR was enhanced by 5-HT at concentrations above 0.01 nM. 7. TPA (100 nM) blocked the GTP gamma S-plus-5-HT-potentiated HRs after the first four to five HRs. Sphingosine (2 microM) augmented the series of HRs.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF


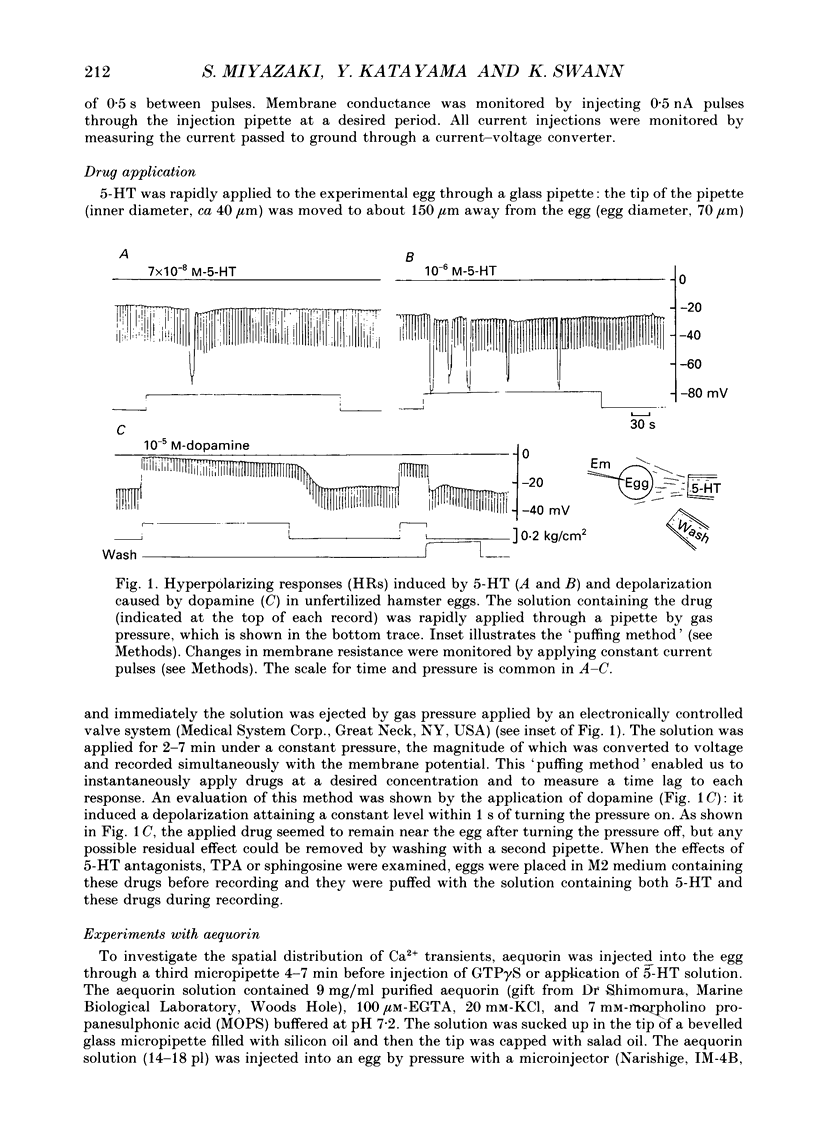
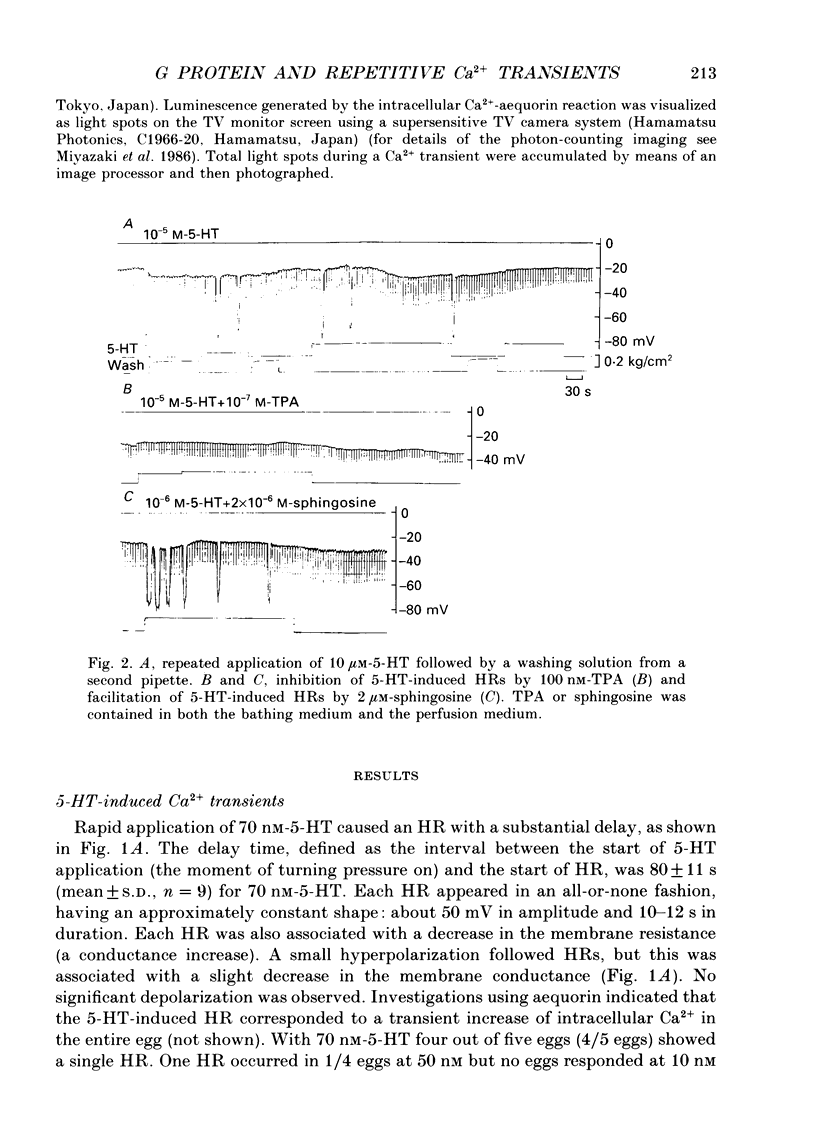
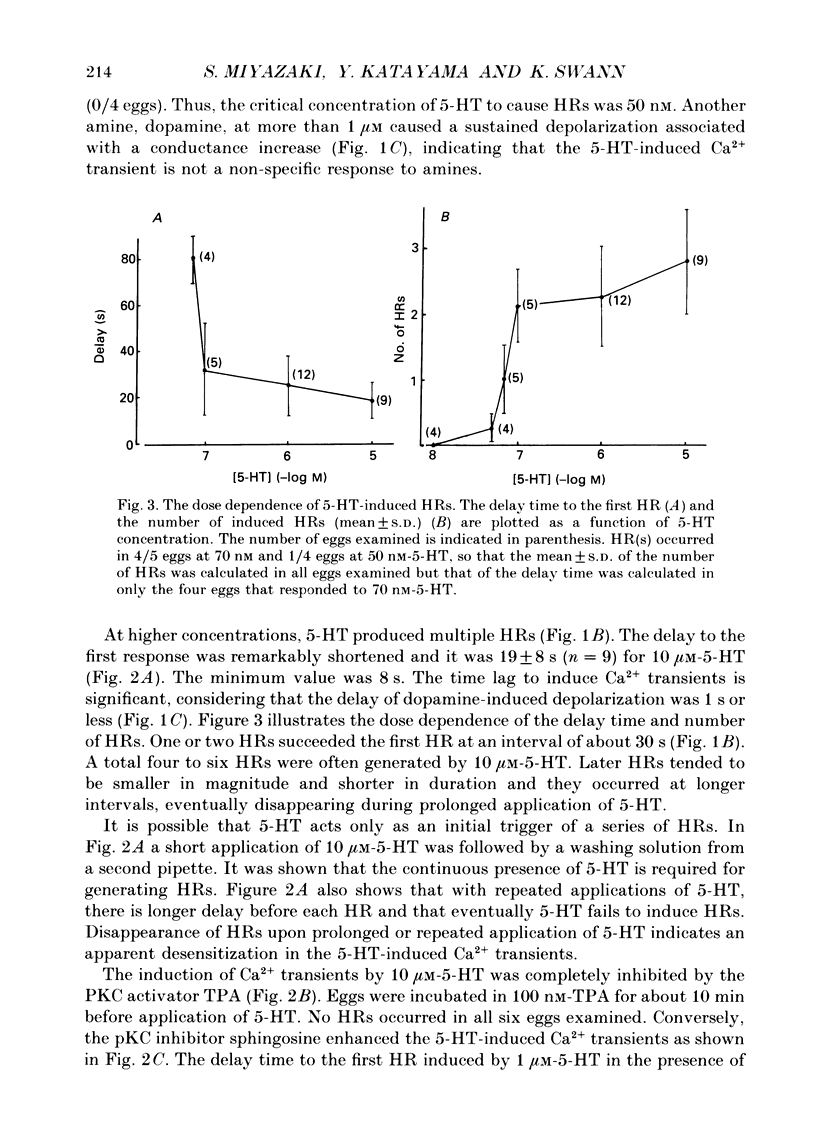
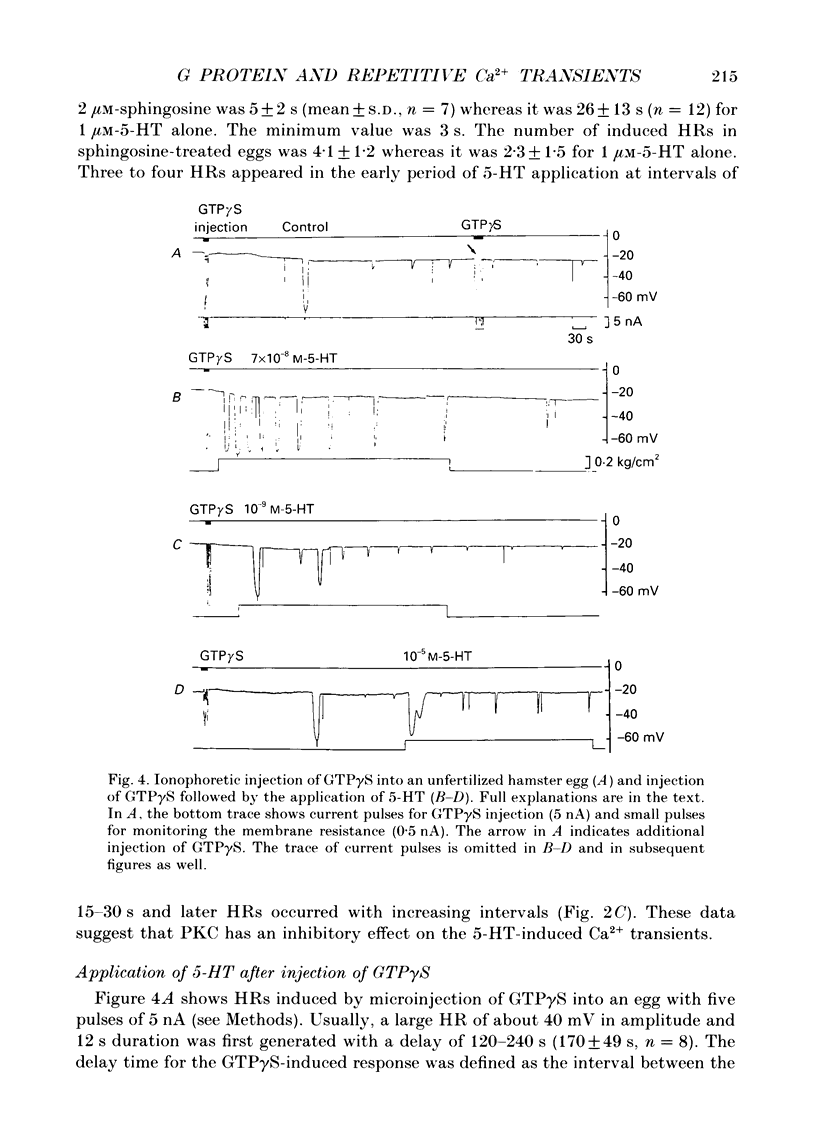

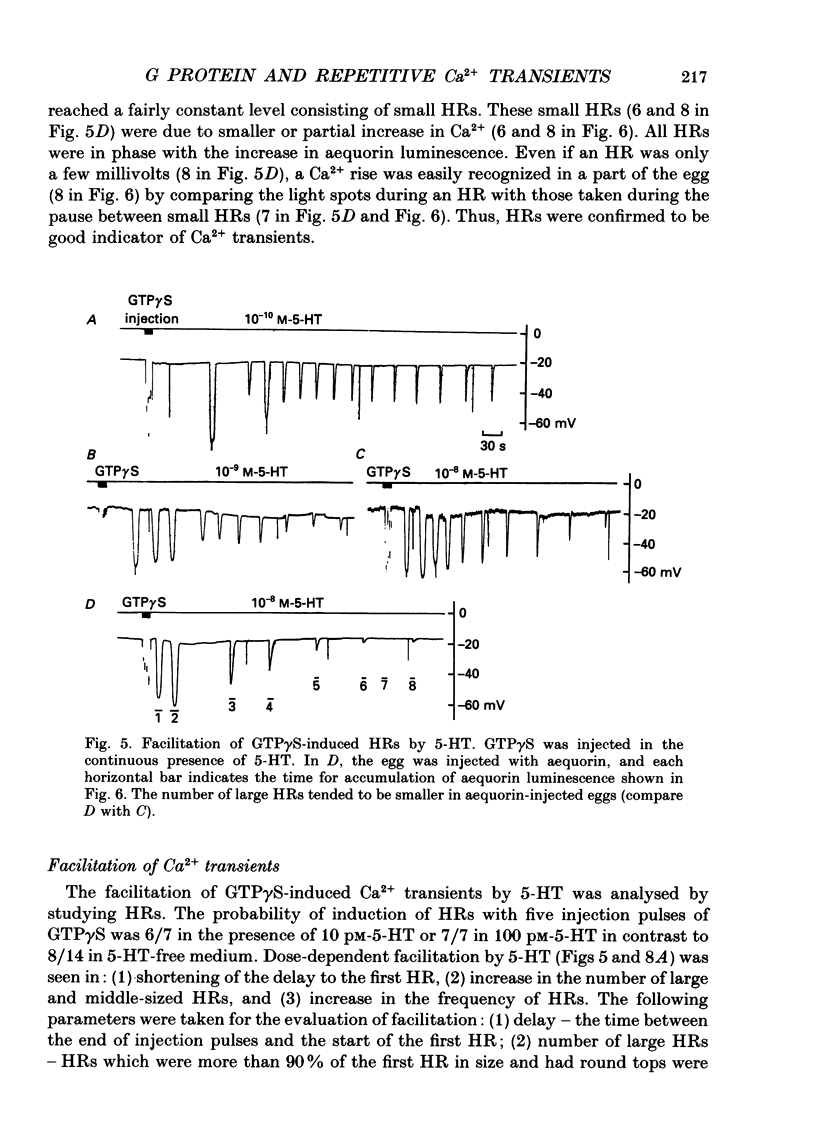
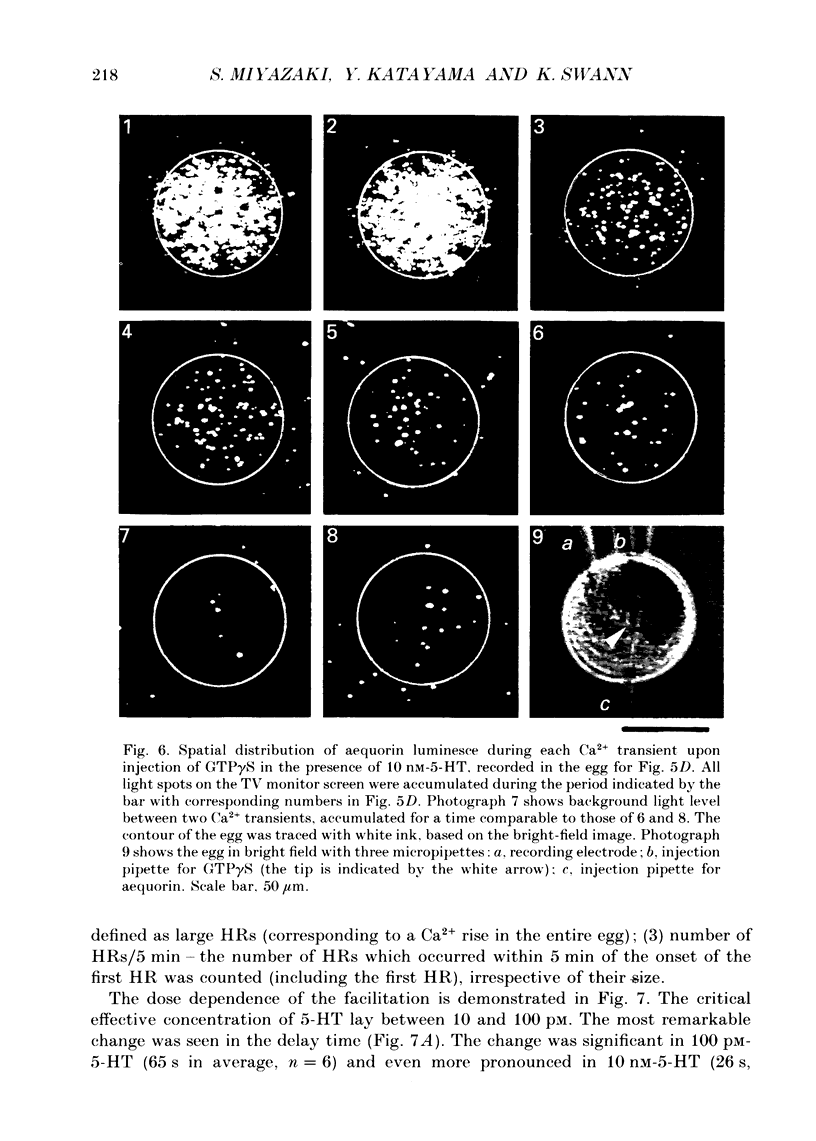
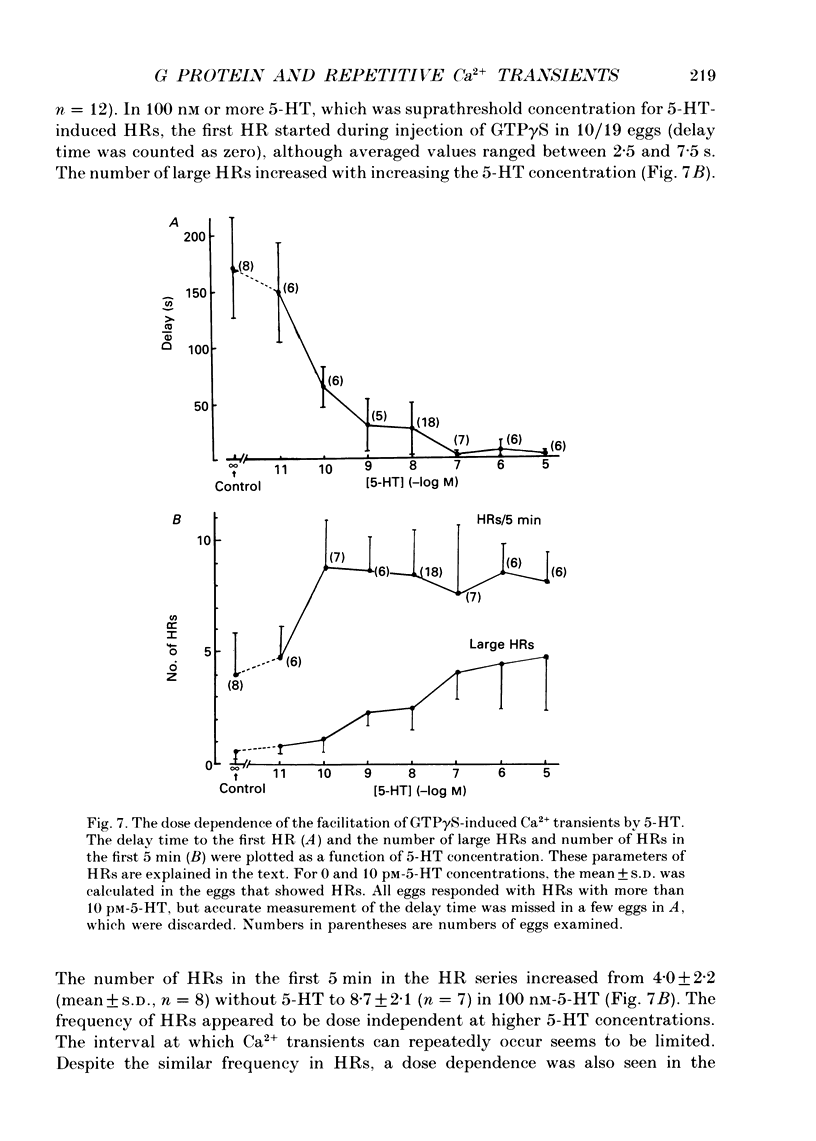
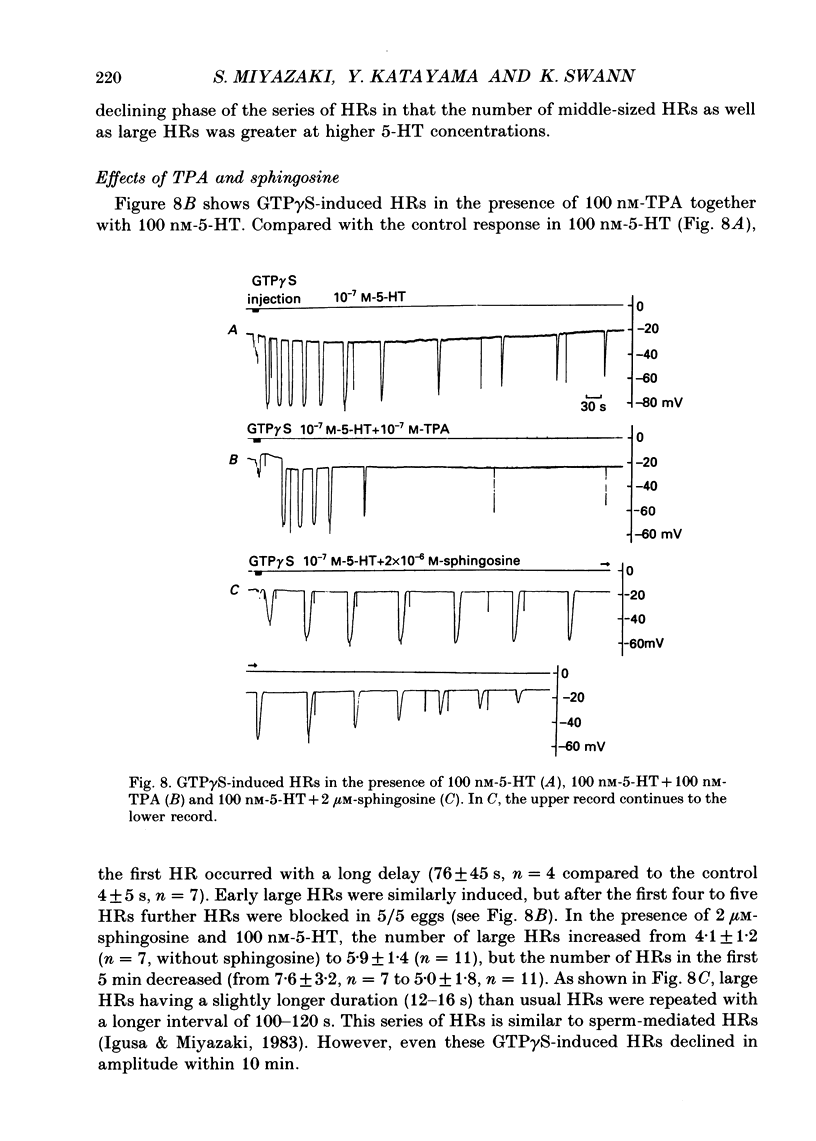
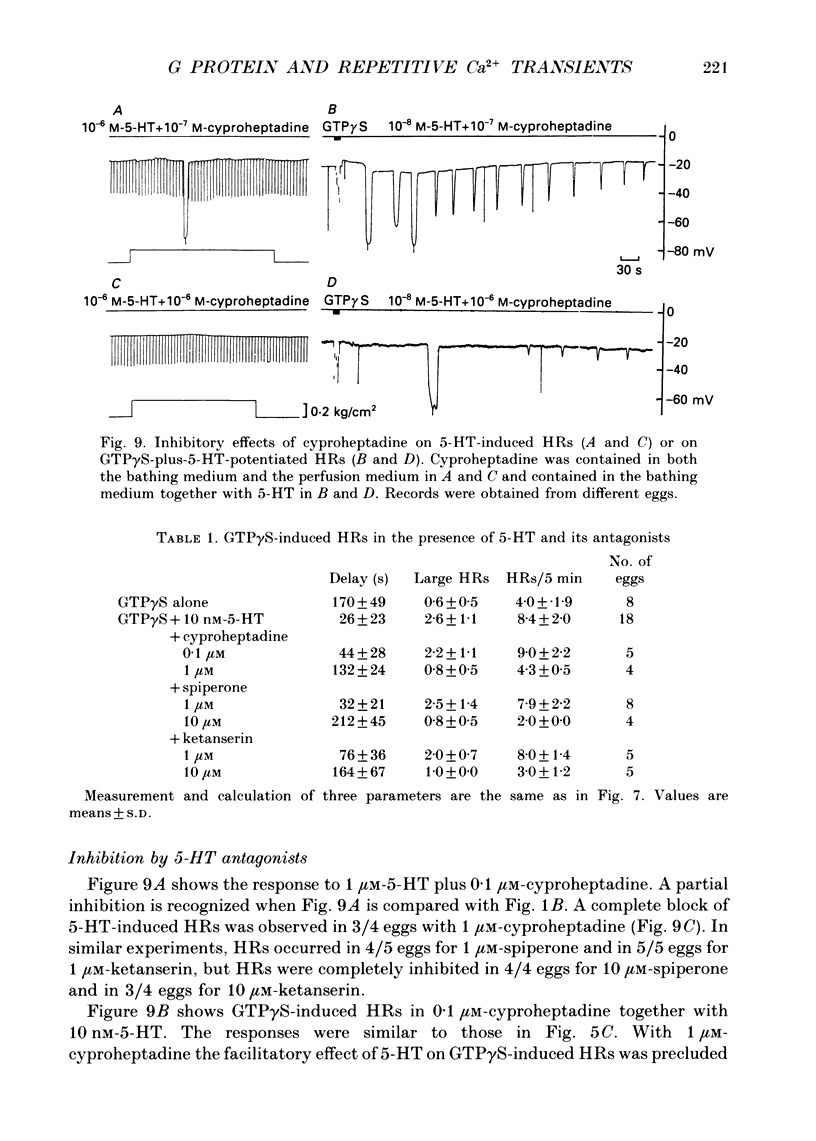
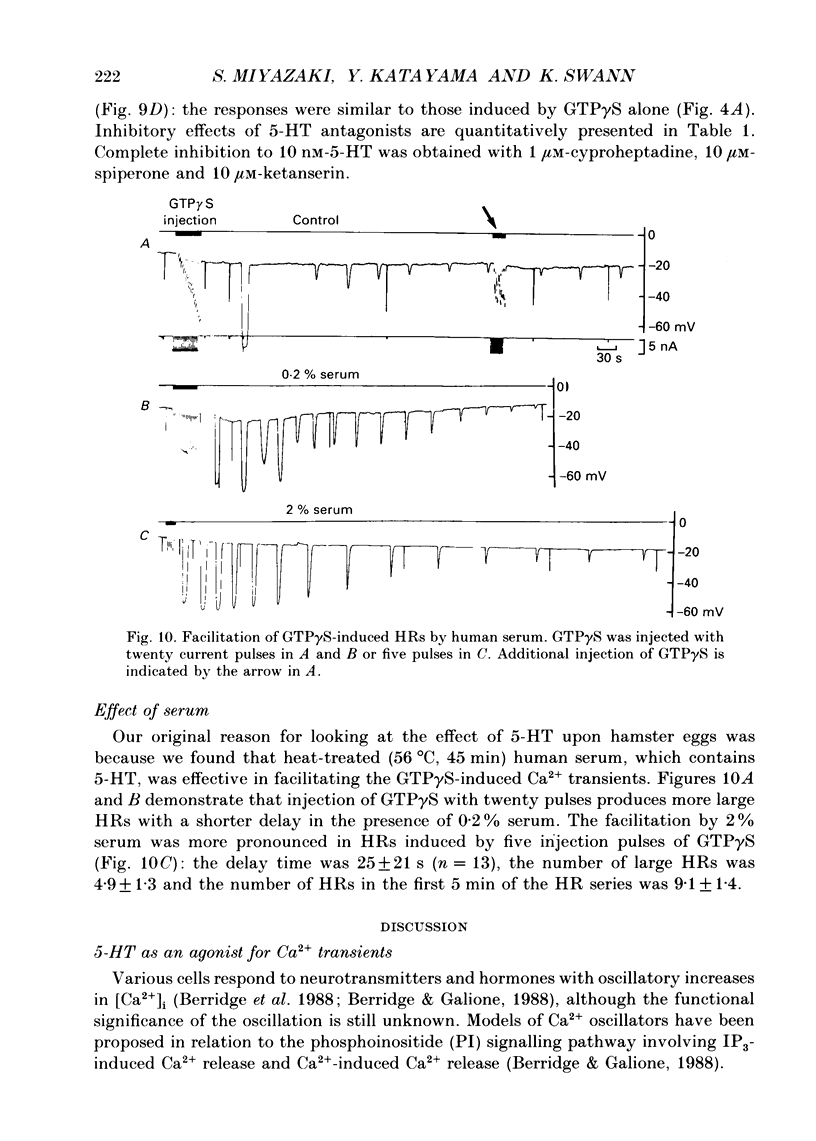





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J., Cobbold P. H., Cuthbertson K. S. Spatial and temporal aspects of cell signalling. Philos Trans R Soc Lond B Biol Sci. 1988 Jul 26;320(1199):325–343. doi: 10.1098/rstb.1988.0080. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Galione A. Cytosolic calcium oscillators. FASEB J. 1988 Dec;2(15):3074–3082. doi: 10.1096/fasebj.2.15.2847949. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Brown K. D., Blakeley D. M., Hamon M. H., Laurie M. S., Corps A. N. Protein kinase C-mediated negative-feedback inhibition of unstimulated and bombesin-stimulated polyphosphoinositide hydrolysis in Swiss-mouse 3T3 cells. Biochem J. 1987 Aug 1;245(3):631–639. doi: 10.1042/bj2450631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochet C., Gill G. N., Meisenhelder J., Cooper J. A., Hunter T. C-kinase phosphorylates the epidermal growth factor receptor and reduces its epidermal growth factor-stimulated tyrosine protein kinase activity. J Biol Chem. 1984 Feb 25;259(4):2553–2558. [PubMed] [Google Scholar]
- Cuthbertson K. S., Whittingham D. G., Cobbold P. H. Free Ca2+ increases in exponential phases during mouse oocyte activation. Nature. 1981 Dec 24;294(5843):754–757. doi: 10.1038/294754a0. [DOI] [PubMed] [Google Scholar]
- ERSPAMER V. Pharmacology of indole-alkylamines. Pharmacol Rev. 1954 Dec;6(4):425–487. [PubMed] [Google Scholar]
- Evans M. G., Marty A. Potentiation of muscarinic and alpha-adrenergic responses by an analogue of guanosine 5'-triphosphate. Proc Natl Acad Sci U S A. 1986 Jun;83(11):4099–4103. doi: 10.1073/pnas.83.11.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton B. P., Whittingham D. G. Activation of mammalian oocytes by intracellular injection of calcium. Nature. 1978 May 11;273(5658):149–151. doi: 10.1038/273149a0. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Igusa Y., Miyazaki S. Effects of altered extracellular and intracellular calcium concentration on hyperpolarizing responses of the hamster egg. J Physiol. 1983 Jul;340:611–632. doi: 10.1113/jphysiol.1983.sp014783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igusa Y., Miyazaki S. Periodic increase of cytoplasmic free calcium in fertilized hamster eggs measured with calcium-sensitive electrodes. J Physiol. 1986 Aug;377:193–205. doi: 10.1113/jphysiol.1986.sp016181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob R., Merritt J. E., Hallam T. J., Rink T. J. Repetitive spikes in cytoplasmic calcium evoked by histamine in human endothelial cells. Nature. 1988 Sep 1;335(6185):40–45. doi: 10.1038/335040a0. [DOI] [PubMed] [Google Scholar]
- Kusano K., Miledi R., Stinnakre J. Cholinergic and catecholaminergic receptors in the Xenopus oocyte membrane. J Physiol. 1982 Jul;328:143–170. doi: 10.1113/jphysiol.1982.sp014257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano I., Marty A. Protein kinase C activators inhibit the inositol trisphosphate-mediated muscarinic current responses in rat lacrimal cells. J Physiol. 1987 Dec;394:239–248. doi: 10.1113/jphysiol.1987.sp016868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch C. J., Charest R., Bocckino S. B., Exton J. H., Blackmore P. F. Inhibition of hepatic alpha 1-adrenergic effects and binding by phorbol myristate acetate. J Biol Chem. 1985 Mar 10;260(5):2844–2851. [PubMed] [Google Scholar]
- Löffelholz K. Receptor regulation of choline phospholipid hydrolysis. A novel source of diacylglycerol and phosphatidic acid. Biochem Pharmacol. 1989 May 15;38(10):1543–1549. doi: 10.1016/0006-2952(89)90299-2. [DOI] [PubMed] [Google Scholar]
- Maruyama Y. Activation and desensitization mechanisms of muscarinic current response in single pancreatic acinar cells of rats. J Physiol. 1989 Oct;417:343–359. doi: 10.1113/jphysiol.1989.sp017805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama Y. Agonist-induced changes in cell membrane capacitance and conductance in dialysed pancreatic acinar cells of rats. J Physiol. 1988 Dec;406:299–313. doi: 10.1113/jphysiol.1988.sp017381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt J. E., Taylor C. W., Rubin R. P., Putney J. W., Jr Evidence suggesting that a novel guanine nucleotide regulatory protein couples receptors to phospholipase C in exocrine pancreas. Biochem J. 1986 Jun 1;236(2):337–343. doi: 10.1042/bj2360337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Parker I. Latencies of membrane currents evoked in Xenopus oocytes by receptor activation, inositol trisphosphate and calcium. J Physiol. 1989 Aug;415:189–210. doi: 10.1113/jphysiol.1989.sp017718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki S., Hashimoto N., Yoshimoto Y., Kishimoto T., Igusa Y., Hiramoto Y. Temporal and spatial dynamics of the periodic increase in intracellular free calcium at fertilization of golden hamster eggs. Dev Biol. 1986 Nov;118(1):259–267. doi: 10.1016/0012-1606(86)90093-x. [DOI] [PubMed] [Google Scholar]
- Miyazaki S., Igusa Y. Ca-mediated activation of a K current at fertilization of golden hamster eggs. Proc Natl Acad Sci U S A. 1982 Feb;79(3):931–935. doi: 10.1073/pnas.79.3.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki S., Igusa Y. Fertilization potential in golden hamster eggs consists of recurring hyperpolarizations. Nature. 1981 Apr 23;290(5808):702–704. doi: 10.1038/290702a0. [DOI] [PubMed] [Google Scholar]
- Miyazaki S. Inositol 1,4,5-trisphosphate-induced calcium release and guanine nucleotide-binding protein-mediated periodic calcium rises in golden hamster eggs. J Cell Biol. 1988 Feb;106(2):345–353. doi: 10.1083/jcb.106.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker I., Gundersen C. B., Miledi R. Intracellular Ca2+-dependent and Ca2+-independent responses of rat brain serotonin receptors transplanted to Xenopus oocytes. Neurosci Res. 1985 Aug;2(6):491–496. doi: 10.1016/0168-0102(85)90021-5. [DOI] [PubMed] [Google Scholar]
- Penner R., Neher E. The role of calcium in stimulus-secretion coupling in excitable and non-excitable cells. J Exp Biol. 1988 Sep;139:329–345. doi: 10.1242/jeb.139.1.329. [DOI] [PubMed] [Google Scholar]
- Peroutka S. J. 5-Hydroxytryptamine receptor subtypes: molecular, biochemical and physiological characterization. Trends Neurosci. 1988 Nov;11(11):496–500. doi: 10.1016/0166-2236(88)90011-2. [DOI] [PubMed] [Google Scholar]
- Renaud F., Parisi E., Capasso A., De Prisco P. On the role of serotonin and 5-methoxy-tryptamine in the regulation of cell division in sea urchin eggs. Dev Biol. 1983 Jul;98(1):37–46. doi: 10.1016/0012-1606(83)90333-0. [DOI] [PubMed] [Google Scholar]
- Rink T. J., Jacob R. Calcium oscillations in non-excitable cells. Trends Neurosci. 1989 Feb;12(2):43–46. doi: 10.1016/0166-2236(89)90133-1. [DOI] [PubMed] [Google Scholar]
- Sagi-Eisenberg R. GTP-binding proteins as possible targets for protein kinase C action. Trends Biochem Sci. 1989 Sep;14(9):355–357. doi: 10.1016/0968-0004(89)90001-7. [DOI] [PubMed] [Google Scholar]
- Sauvé R., Simoneau C., Parent L., Monette R., Roy G. Oscillatory activation of calcium-dependent potassium channels in HeLa cells induced by histamine H1 receptor stimulation: a single-channel study. J Membr Biol. 1987;96(3):199–208. doi: 10.1007/BF01869302. [DOI] [PubMed] [Google Scholar]
- Swann K., Igusa Y., Miyazaki S. Evidence for an inhibitory effect of protein kinase C on G-protein-mediated repetitive calcium transients in hamster eggs. EMBO J. 1989 Dec 1;8(12):3711–3718. doi: 10.1002/j.1460-2075.1989.tb08546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods N. M., Cuthbertson K. S., Cobbold P. H. Agonist-induced oscillations in cytoplasmic free calcium concentration in single rat hepatocytes. Cell Calcium. 1987 Feb;8(1):79–100. doi: 10.1016/0143-4160(87)90038-8. [DOI] [PubMed] [Google Scholar]
- Woods N. M., Cuthbertson K. S., Cobbold P. H. Phorbol-ester-induced alterations of free calcium ion transients in single rat hepatocytes. Biochem J. 1987 Sep 15;246(3):619–623. doi: 10.1042/bj2460619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yada T., Oiki S., Ueda S., Okada Y. Synchronous oscillation of the cytoplasmic Ca2+ concentration and membrane potential in cultured epithelial cells (Intestine 407). Biochim Biophys Acta. 1986 Jun 16;887(1):105–112. doi: 10.1016/0167-4889(86)90129-1. [DOI] [PubMed] [Google Scholar]
- Yanagimachi R. In vitro capacitation of golden hamster spermatozoa by homologous and heterologous blood sera. Biol Reprod. 1970 Oct;3(2):147–153. doi: 10.1093/biolreprod/3.2.147. [DOI] [PubMed] [Google Scholar]
- Yanagimachi R. In vitro capacitation of hamster spermatozoa by follicular fluid. J Reprod Fertil. 1969 Mar;18(2):275–286. doi: 10.1530/jrf.0.0180275. [DOI] [PubMed] [Google Scholar]



