Abstract
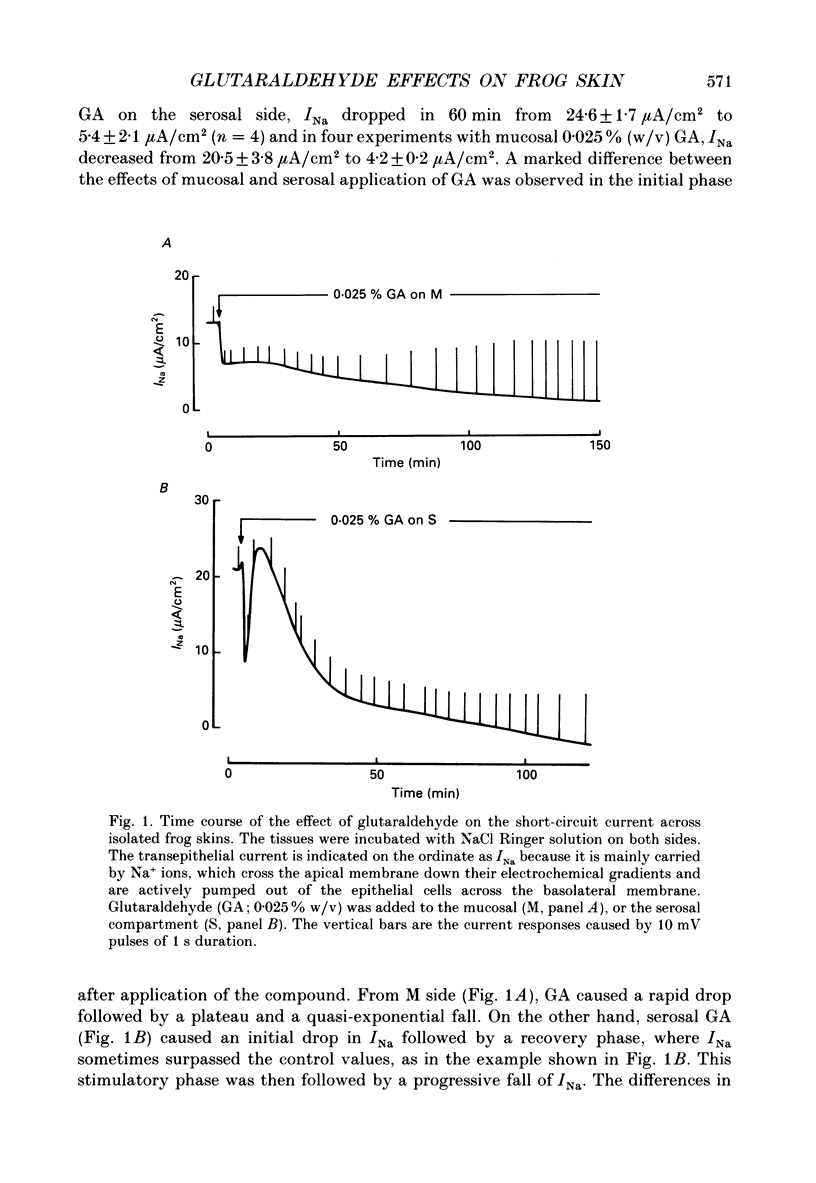
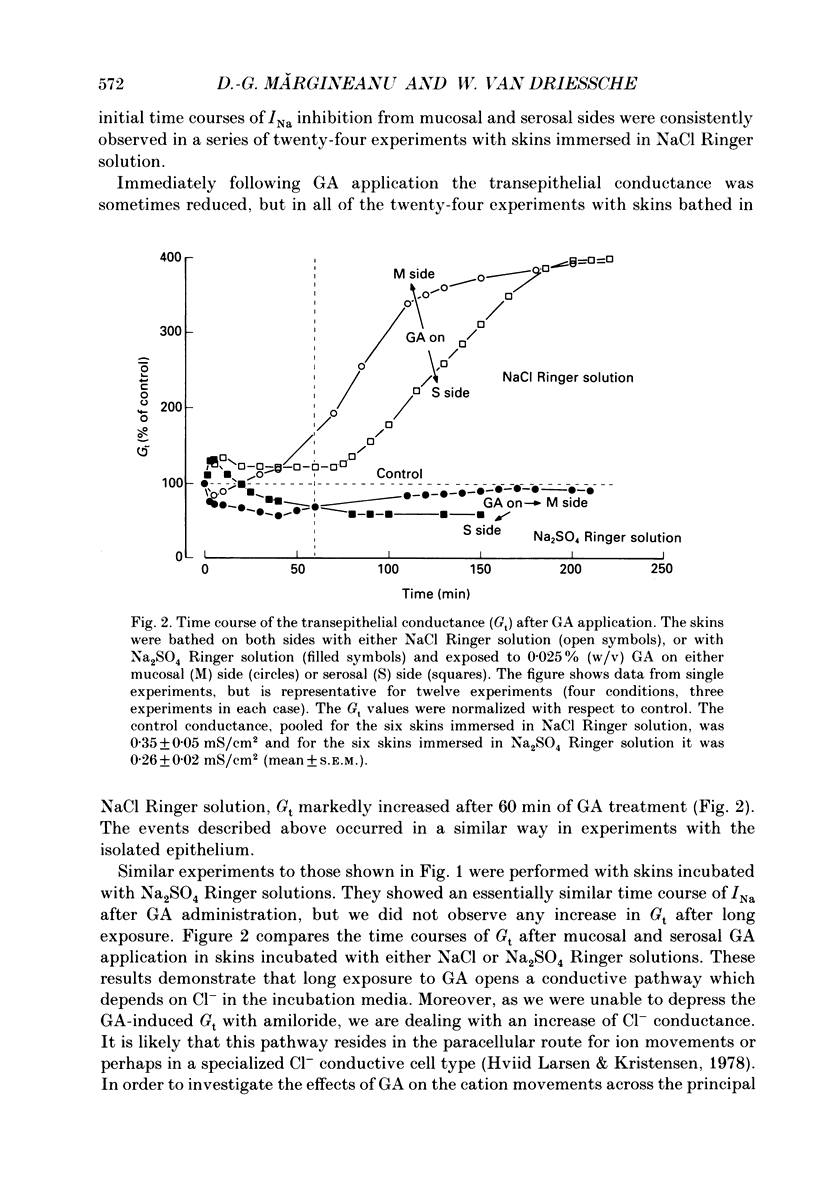
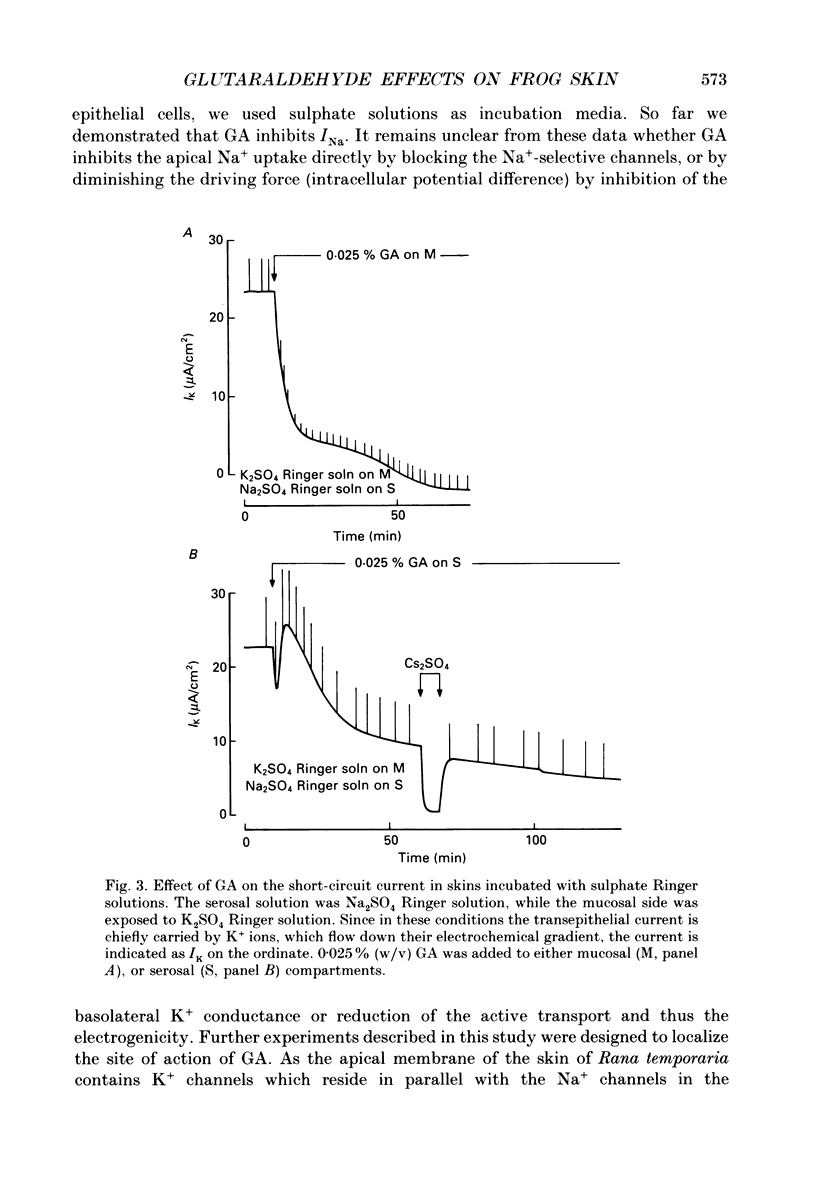
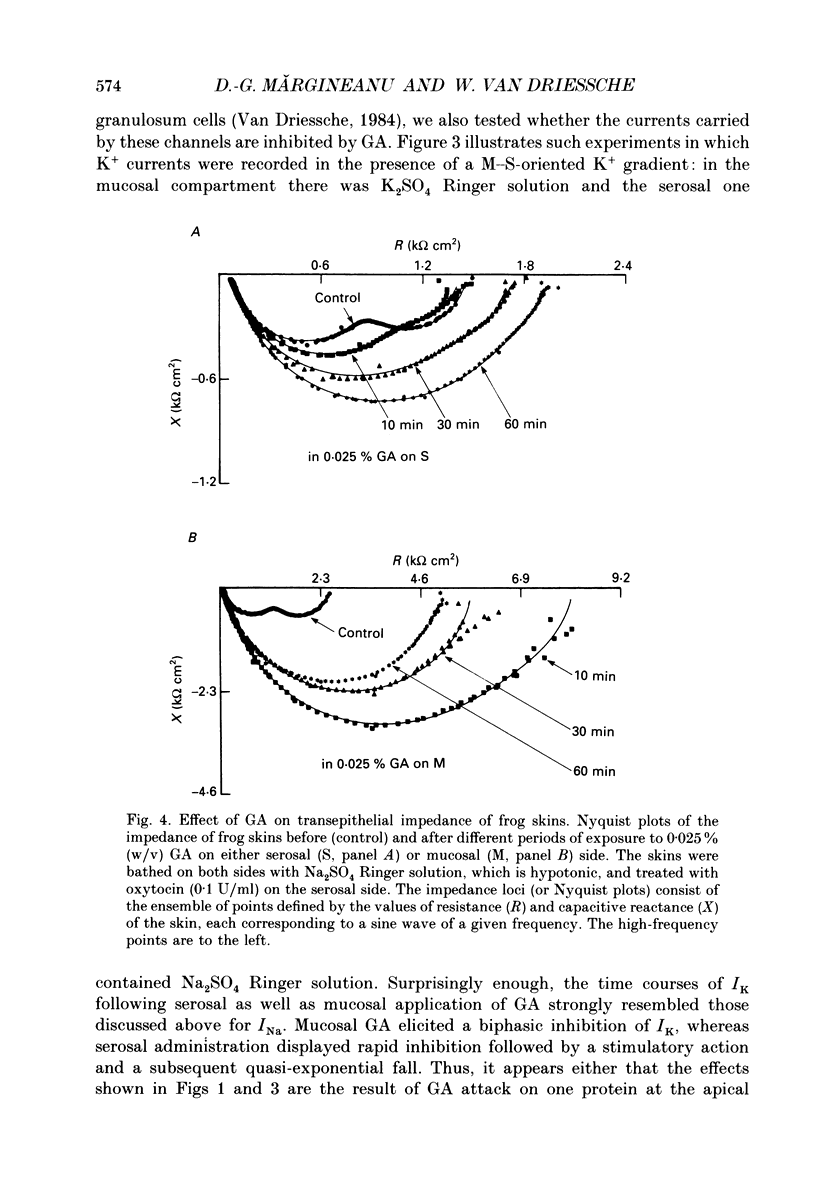
1. The effects of millimolar concentrations of glutaraldehyde on the electrophysiological properties of the epithelium of frog skin (Rana temporaria) were investigated. We recorded short-circuit current (Isc), transepithelial conductance (Gt) and impedance (Zt), fractional resistance (fRo) and the potential difference across the apical membrane (Vo). We used either Na+ or K+ as major mucosal cations to compare the effects on transepithelial Na+ and K+ currents (INa and IK) and thus on the apical Na+ and K+ permeabilities. 2. At concentrations above 0.005% (w/v) or 0.5 mM, glutaraldehyde irreversibly and completely inhibits both INa and IK within 2-3 h. The initial time courses of the inhibition of transepithelial currents following serosal and mucosal applications of the compound markedly differ. 3. Glutaraldehyde decreased Gt in sulphate Ringer solutions while it augmented Gt severalfold in chloride Ringer solution. 4. Measurements of the transepithelial impedance of tissues incubated with sulphate solutions showed that glutaraldehyde increased the resistances of both apical and basolateral membranes significantly. The capacitance of the apical membrane was augmented, while the basolateral membrane capacitance was drastically decreased. 5. Microelectrode impalements of the granulosum cells showed that glutaraldehyde decreased Vo by more than 40 mV and increased fRo, which reached values around 90%. 6. The role of free amino groups in ion-transporting proteins and the potential non-fixative uses of protein cross-linkers in epithelia are discussed.
Full text
PDF



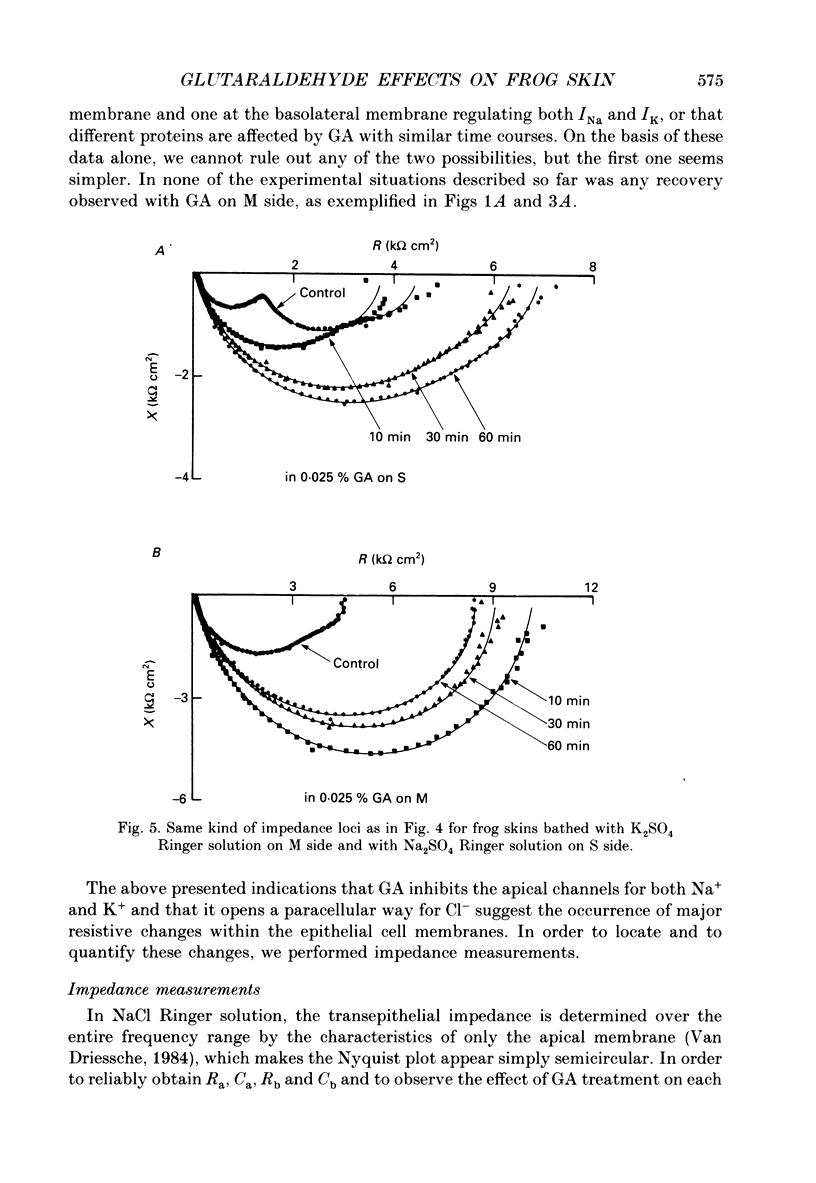
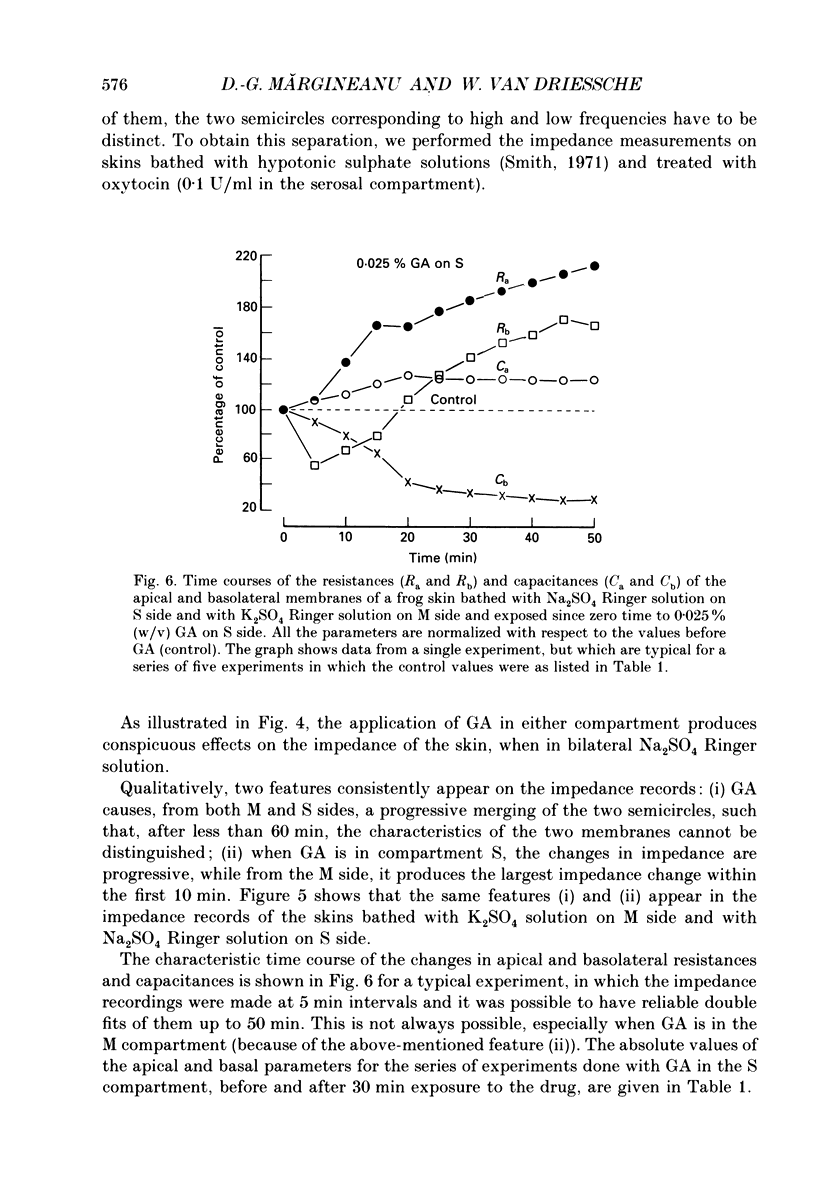
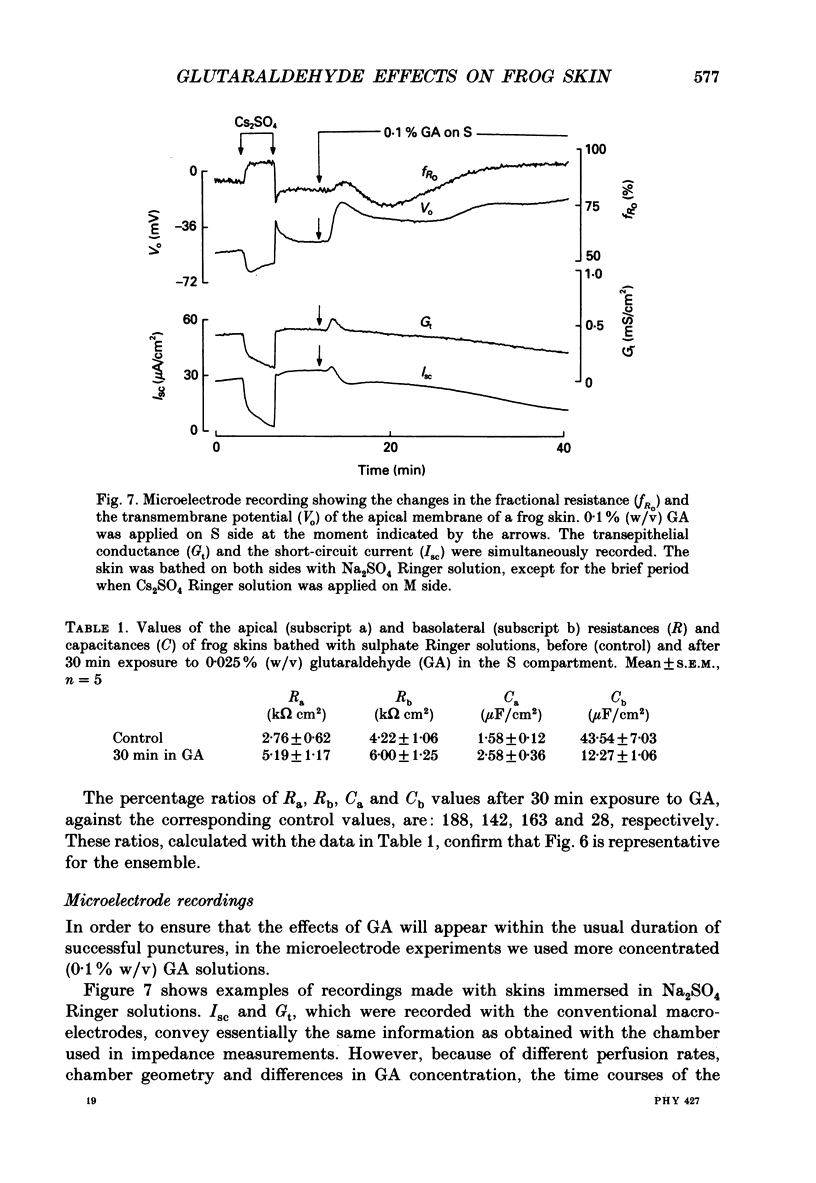




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aboulafia J., Lacaz-Vieira F. Hydrosmotic salt effect in toad skin: urea permeability and glutaraldehyde fixation of water channels. J Membr Biol. 1985;87(3):249–252. doi: 10.1007/BF01871225. [DOI] [PubMed] [Google Scholar]
- Corry W. D., Meiselman H. J. Modification of erythrocyte physicochemical properties by millimolar concentrations of glutaraldehyde. Blood Cells. 1978;4(3):465–483. [PubMed] [Google Scholar]
- De Wolf I., Van Driessche W. Voltage-dependent Ba2+ block of K+ channels in apical membrane of frog skin. Am J Physiol. 1986 Nov;251(5 Pt 1):C696–C706. doi: 10.1152/ajpcell.1986.251.5.C696. [DOI] [PubMed] [Google Scholar]
- Eggena P. Effect of glutaraldehyde on hydrosmotic response of toad bladder to vasopressin. Am J Physiol. 1983 Jan;244(1):C37–C43. doi: 10.1152/ajpcell.1983.244.1.C37. [DOI] [PubMed] [Google Scholar]
- Fisher R. S., Erlij D., Helman S. I. Intracellular voltage of isolated epithelia of frog skin: apical and basolateral cell punctures. J Gen Physiol. 1980 Oct;76(4):447–453. doi: 10.1085/jgp.76.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn R., Brodwick M. S., Eaton D. C. Effect of protein cross-linking reagents on membrane currents of squid axon. Am J Physiol. 1980 Mar;238(3):C127–C132. doi: 10.1152/ajpcell.1980.238.3.C127. [DOI] [PubMed] [Google Scholar]
- Hviid Larsen E., Kristensen P. Properties of a conductive cellular chloride pathway in the skin of the toad (Bufo bufo). Acta Physiol Scand. 1978 Jan;102(1):1–21. doi: 10.1111/j.1748-1716.1978.tb06041.x. [DOI] [PubMed] [Google Scholar]
- Kondo Y., Imai M. Effect of glutaraldehyde on renal tubular function. II. Selective inhibition of Cl- transport in the hamster thin ascending limb of Henle's loop. Pflugers Arch. 1987 May;408(5):484–490. doi: 10.1007/BF00585073. [DOI] [PubMed] [Google Scholar]
- Korn A. H., Feairheller S. H., Filachione E. M. Glutaraldehyde: nature of the reagent. J Mol Biol. 1972 Apr 14;65(3):525–529. doi: 10.1016/0022-2836(72)90206-9. [DOI] [PubMed] [Google Scholar]
- Mărgineanu D. G., Katona E., Popa J. Kinetics of nerve impulse blocking by protein cross-linking aldehydes. Apparent critical thermal points. Biochim Biophys Acta. 1981 Dec 21;649(3):581–586. doi: 10.1016/0005-2736(81)90162-0. [DOI] [PubMed] [Google Scholar]
- Mărgineanu D. G., Rucăreanu C., Flonta M. L., Finichiu D. Glutaraldehyde inhibits the active transport of sodium and the oxygen consumption, while increasing the water diffusional permeability in frog skin. Arch Int Physiol Biochim. 1984 Nov;92(4):305–312. doi: 10.3109/13813458409071171. [DOI] [PubMed] [Google Scholar]
- Nagel W. Effects of antidiuretic hormone upon electrical potential and resistance of apical and basolateral membranes of frog skin. J Membr Biol. 1978 Sep 18;42(2):99–122. doi: 10.1007/BF01885366. [DOI] [PubMed] [Google Scholar]
- Reichlin M. Use of glutaraldehyde as a coupling agent for proteins and peptides. Methods Enzymol. 1980;70(A):159–165. doi: 10.1016/s0076-6879(80)70047-2. [DOI] [PubMed] [Google Scholar]
- Smith P. G. The low-frequency electrical impedance of the isolated frog skin. Acta Physiol Scand. 1971 Mar;81(3):355–366. doi: 10.1111/j.1748-1716.1971.tb04910.x. [DOI] [PubMed] [Google Scholar]
- Van Driessche W., Lindemann B. Low-noise amplification of voltage and current fluctuations arising in epithelia. Rev Sci Instrum. 1978 Jan;49(1):52–52. doi: 10.1063/1.1135251. [DOI] [PubMed] [Google Scholar]
- Van Driessche W. Physiological role of apical potassium ion channels in frog skin. J Physiol. 1984 Nov;356:79–95. doi: 10.1113/jphysiol.1984.sp015454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Driessche W., Zeiske W. Ionic channels in epithelial cell membranes. Physiol Rev. 1985 Oct;65(4):833–903. doi: 10.1152/physrev.1985.65.4.833. [DOI] [PubMed] [Google Scholar]


