Abstract
1. The relationship between slowing of relaxation and changes of intracellular pH and phosphorous metabolites has been examined in human skeletal muscle during the development of fatigue and subsequent recovery. Results obtained with normal subjects have been compared with those from a subject with myophosphorylase deficiency (MPD) who produced no H+ from glycolysis during exercise and therefore afforded the opportunity of assessing the role of H+ in the slowing of relaxation. 2. Subjects fatigued the first dorsal interosseous muscle in a stepwise fashion under ischaemic conditions, with intervals between the fatiguing contractions during which the relaxation rate was measured from brief tetanic contractions and the muscle phosphorous metabolites and pH were measured by nuclear magnetic resonance spectroscopy. 3. After 21 s maximal voluntary contraction under ischaemic conditions, relaxation in the MPD subject slowed to approximately 50% of the rate in the fresh muscle at a time when the intramuscular pH had not changed. This demonstrates that there is a mechanism causing slowing of relaxation that is independent of H+ accumulation. 4. The normal subjects showed a slow recovery of relaxation compared to the MPD subject when the circulation was restored. The main difference in the intracellular metabolite concentrations between MPD and normal subjects at this time was that, for the latter, the pH remained low (around 6.5) for at least 60 s after the circulation was restored. The results suggest that the slow recovery is a consequence of continuing acidosis, i.e. the existence of a pH-dependent mechanism of slowing. 5. The existence of a pH-dependent mechanism was further indicated by the fact that for the normal subjects, for a similar intracellular concentration of phosphocreatine, relaxation of the recovering muscle was approximately half that of the fatiguing muscle. This was at a time when the pH of the recovering muscle was 0.3-0.4 units less than in the partially fatigued muscle. 6. The results show that in normal muscle there are at least two processes that lead to slow relaxation in fatigued muscle: one due to H+ accumulation, the other being independent of H+.
Full text
PDF



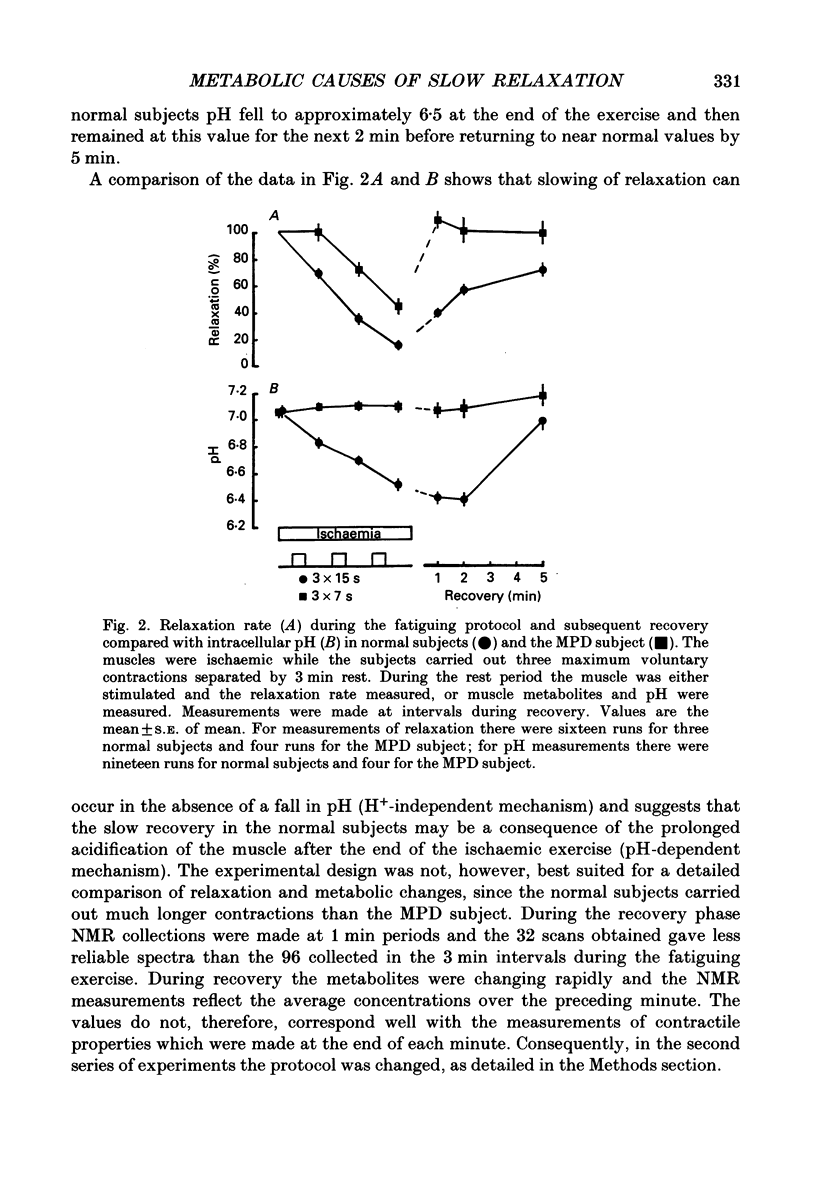
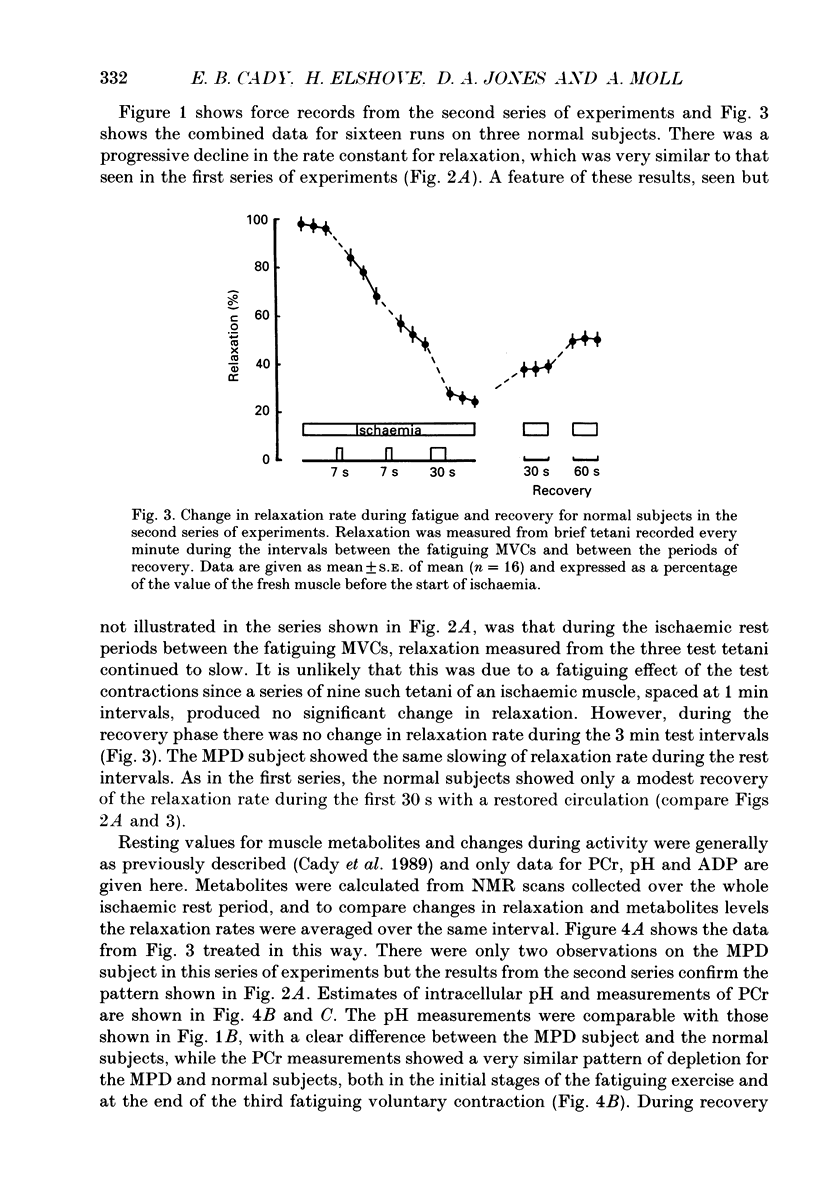
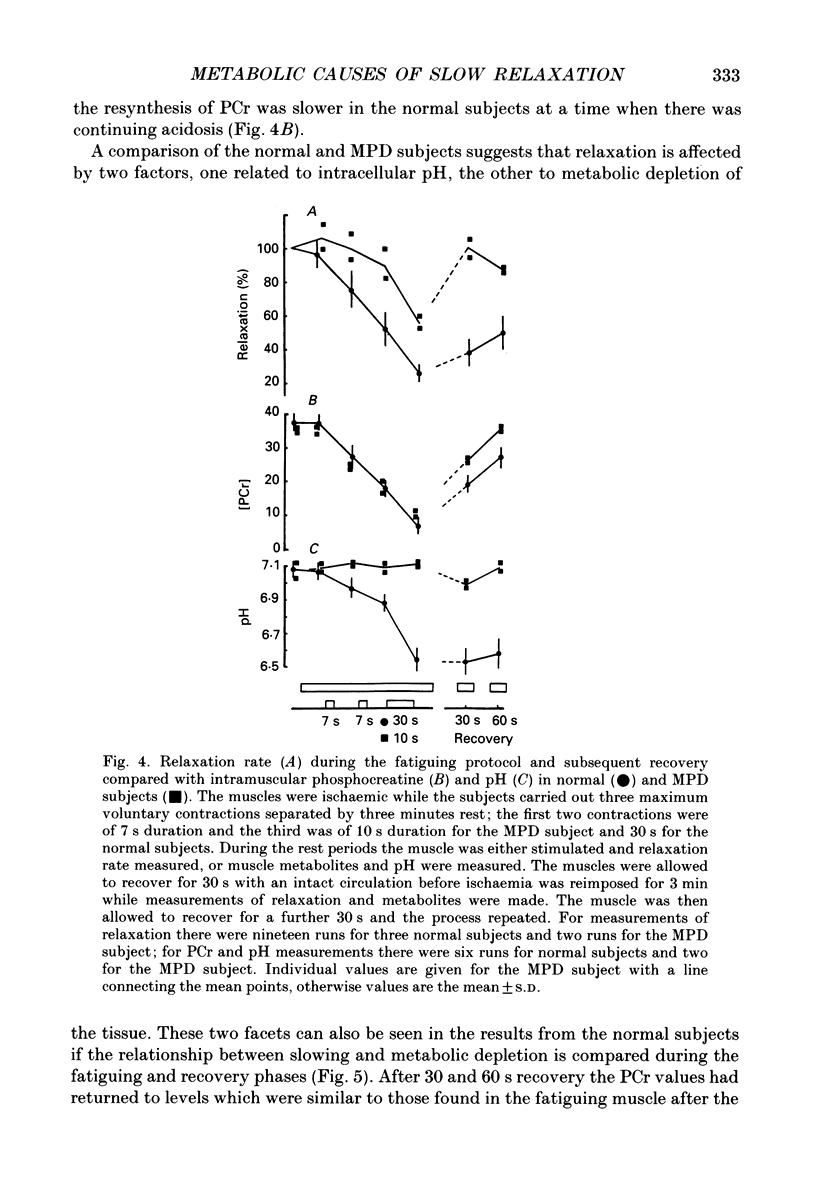



Selected References
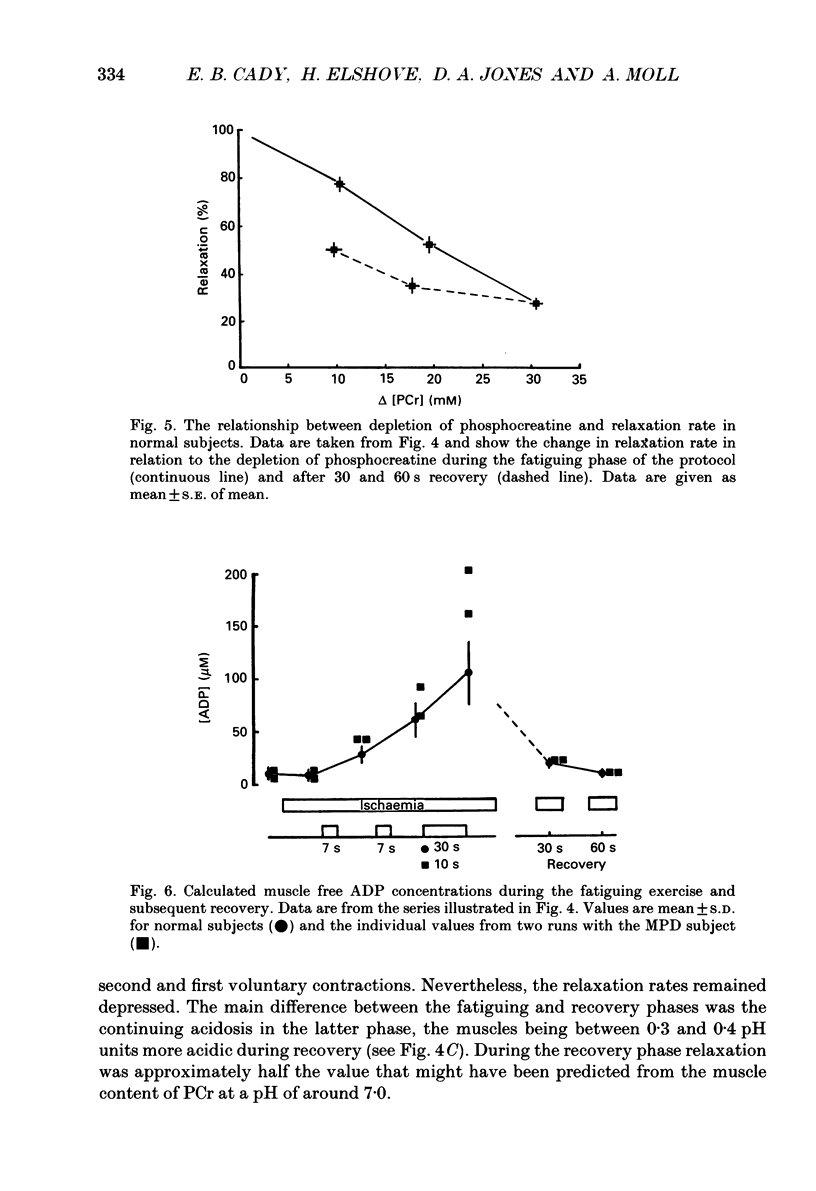
These references are in PubMed. This may not be the complete list of references from this article.
- Blinks J. R., Rüdel R., Taylor S. R. Calcium transients in isolated amphibian skeletal muscle fibres: detection with aequorin. J Physiol. 1978 Apr;277:291–323. doi: 10.1113/jphysiol.1978.sp012273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cady E. B., Jones D. A., Lynn J., Newham D. J. Changes in force and intracellular metabolites during fatigue of human skeletal muscle. J Physiol. 1989 Nov;418:311–325. doi: 10.1113/jphysiol.1989.sp017842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase P. B., Kushmerick M. J. Effects of pH on contraction of rabbit fast and slow skeletal muscle fibers. Biophys J. 1988 Jun;53(6):935–946. doi: 10.1016/S0006-3495(88)83174-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R., Pate E. The effects of ADP and phosphate on the contraction of muscle fibers. Biophys J. 1985 Nov;48(5):789–798. doi: 10.1016/S0006-3495(85)83837-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow M. T., Kushmerick M. J. Correlated reduction of velocity of shortening and the rate of energy utilization in mouse fast-twitch muscle during a continuous tetanus. J Gen Physiol. 1983 Nov;82(5):703–720. doi: 10.1085/jgp.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson M. J., Gadian D. G., Wilkie D. R. Mechanical relaxation rate and metabolism studied in fatiguing muscle by phosphorus nuclear magnetic resonance. J Physiol. 1980 Feb;299:465–484. doi: 10.1113/jphysiol.1980.sp013137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A., Mattiazzi A. R. Effects of fatigue and altered pH on isometric force and velocity of shortening at zero load in frog muscle fibres. J Muscle Res Cell Motil. 1981 Sep;2(3):321–334. doi: 10.1007/BF00713270. [DOI] [PubMed] [Google Scholar]
- Edwards R. H., Hill D. K., Jones D. A. Metabolic changes associated with the slowing of relaxation in fatigued mouse muscle. J Physiol. 1975 Oct;251(2):287–301. doi: 10.1113/jphysiol.1975.sp011093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Effects of pH on the myofilaments and the sarcoplasmic reticulum of skinned cells from cardiace and skeletal muscles. J Physiol. 1978 Mar;276:233–255. doi: 10.1113/jphysiol.1978.sp012231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JEWELL B. R., WILKIE D. R. The mechanical properties of relaxing muscle. J Physiol. 1960 Jun;152:30–47. doi: 10.1113/jphysiol.1960.sp006467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lännergren J., Westerblad H. Maximum tension and force-velocity properties of fatigued, single Xenopus muscle fibres studied by caffeine and high K+. J Physiol. 1989 Feb;409:473–490. doi: 10.1113/jphysiol.1989.sp017508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson J. L. The effect of activity on the form of the muscle twitch. J Physiol. 1933 Apr 13;78(1):106–112. doi: 10.1113/jphysiol.1933.sp002990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaud J. M., Allard Y., Mainwood G. W. Is the change in intracellular pH during fatigue large enough to be the main cause of fatigue? Can J Physiol Pharmacol. 1986 Jun;64(6):764–767. doi: 10.1139/y86-130. [DOI] [PubMed] [Google Scholar]
- Sahlin K., Edström L., Sjöholm H., Hultman E. Effects of lactic acid accumulation and ATP decrease on muscle tension and relaxation. Am J Physiol. 1981 Mar;240(3):C121–C126. doi: 10.1152/ajpcell.1981.240.3.C121. [DOI] [PubMed] [Google Scholar]
- Taylor D. J., Styles P., Matthews P. M., Arnold D. A., Gadian D. G., Bore P., Radda G. K. Energetics of human muscle: exercise-induced ATP depletion. Magn Reson Med. 1986 Feb;3(1):44–54. doi: 10.1002/mrm.1910030107. [DOI] [PubMed] [Google Scholar]
- de Haan A., Jones D. A., Sargeant A. J. Changes in velocity of shortening, power output and relaxation rate during fatigue of rat medial gastrocnemius muscle. Pflugers Arch. 1989 Feb;413(4):422–428. doi: 10.1007/BF00584493. [DOI] [PubMed] [Google Scholar]


